Abstract
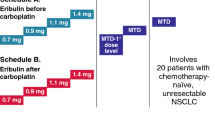
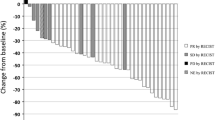
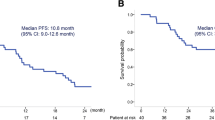
Introduction One standard of care for advanced non–small cell lung cancer (NSCLC) is paclitaxel plus carboplatin ± bevacizumab. This two-step phase I study evaluated the feasibility of adding everolimus to paclitaxel plus carboplatin ± bevacizumab for advanced NSCLC. Methods Adults with advanced NSCLC naive to systemic therapy were enrolled. A Bayesian dose-escalation model was used to identify feasible daily or weekly everolimus doses given with paclitaxel (200 mg/m2 q21 days) and carboplatin (AUC 6 mg/mL/min q21 days) (step 1) and paclitaxel (200 mg/m2 q21 days), carboplatin (AUC 6 mg/mL/min q21 days), and bevacizumab (15 mg/kg q21 days) (step 2). Primary endpoint was end-of-cycle 1 dose-limiting toxicity (DLT) rate. Secondary endpoints included safety; relative dose intensities of paclitaxel, carboplatin, and bevacizumab; pharmacokinetics; and tumor response. Results Fifty-two patients were enrolled and received everolimus 5 mg/day plus carboplatin and paclitaxel (step 1 daily; n = 13); everolimus 30 mg/week plus carboplatin and paclitaxel (step 1 weekly; n = 13); everolimus 5 mg/day plus carboplatin, paclitaxel, and bevacizumab (step 2 daily; n = 13); or everolimus 30 mg/week plus carboplatin, paclitaxel, and bevacizumab (step 2 weekly; n = 13). End-of-cycle 1 DLT rate was 16.7 % (step 1 daily), 30.8 % (step 1 weekly), 30.0 % (step 2 daily), and 16.7 % (step 2 weekly). Cycle 1 DLTs were grade 3 neutropenia, anal abscess, diarrhea, and thrombocytopenia and grade 4 myalgia, cellulitis, neutropenia, febrile neutropenia, pulmonary embolism, and thrombocytopenia. The most common adverse events were neutropenia, fatigue, anemia, and thrombocytopenia. One patient (step 2 daily) experienced complete response, 10 patients partial response. Conclusions The feasible everolimus doses given with carboplatin and paclitaxel ± bevacizumab were 5 mg/day and 30 mg/week. Neither schedule was very well tolerated in this unselected NSCLC population.

Similar content being viewed by others
References
National Comprehensive Cancer Network (2011) Nccn clinical practice guidelines in oncology: non-small cell lung cancer, Version 2.2012
Ramalingam S, Ab S (2006) Salvage therapy for advanced non-small cell lung cancer: factors influencing treatment selection. Oncologist 11:655–665
Behera M, Tk O, Chen Z, Sa K, Fr K, Cp B, Ss R (2012) Single agent maintenance therapy for advanced stage non-small cell lung cancer: a meta-analysis. Lung Cancer 77:331–338
Ma B, Pj H (2004) The tor pathway: a target for cancer therapy. Nat Rev Cancer 4:335–348
Beuvink I, Boulay A, Fumagalli S, Zilbermann F, Ruetz S, O’reilly T, Natt F, Hall J, Ha L, Thomas G (2005) The Mtor inhibitor Rad001 sensitizes tumor cells to Dna-damaged induced apoptosis through inhibition Of P21 translation. Cell 120:747–759
Boulay A, Zumstein-Mecker S, Stephan C, Beuvink I, Zilbermann F, Haller R, Tobler S, Heusser C, O’reilly T, Stolz B, Marti A, Thomas G, Ha L (2004) Antitumor efficacy of intermittent treatment schedules with the rapamycin derivative Rad001 correlates with prolonged inactivation of ribosomal protein S6 kinase 1 in peripheral blood mononuclear cells. Cancer Res 64:252–261
Mabuchi S, Da A, Dc C, Klein-Szanto A, Litwin S, Mk H, Hensley H, Tc H, Jr T (2007) Rad001 (Everolimus) delays tumor onset and progression in a transgenic mouse model of ovarian cancer. Cancer Res 67:2408–2413
La Monica S, Galetti M, Rr A, Cavazzoni A, Ardizzoni A, Tiseo M, Capelletti M, Goldoni M, Tagliaferri S, Mutti A, Fumarola C, Bonelli M, Generali D, Pg P (2009) Everolimus restores gefitinib sensitivity in resistant non-small cell lung cancer cell lines. Biochem Pharmacol 78:460–468
Nakachi I, Naoki K, Soejima K, Kawada I, Watanabe H, Yasuda H, Nakayama S, Yoda S, Satomi R, Ikemura S, Terai H, Sato T, Ishizaka A (2010) The combination of multiple receptor tyrosine kinase inhibitor and mammalian target of rapamycin inhibitor overcomes erlotinib resistance in lung cancer cell lines through C-Met inhibition. Mol Cancer Res 8:1142–1151
Cx X, Li Y, Yue P, Tk O, Ss R, Fr K, Sy S (2011) The combination of Rad001 and Nvp-Bez235 exerts synergistic anticancer activity against non-small cell lung cancer in vitro and in vivo. PLoS One 6, E20899
Afinitor (Everolimus) Tablets For Oral Administration [Prescribing Information] (2012) East Hanover, Nj, Usa: Novartis Pharmaceuticals Corporation
Afinitor Summary Of Product Characteristics (2012) West Sussex, United Kingdom: Novartis Europharm Limited
Votubia Summary Of Product Characteristics (2012) West Sussex, United Kingdom: Novartis Europharm Limited
Baselga J, Campone M, Piccart M, Ha B, Hs R, Sahmoud T, Noguchi S, Gnant M, Ki P, Lebrun F, Jt B, Ito Y, Yardley D, Deleu I, Perez A, Bachelot T, Vittori L, Xu Z, Mukhopadhyay P, Lebwohl D, Gn H (2012) Everolimus in postmenopausal hormone receptor-positive advanced breast cancer. N Engl J Med 366:520–529
Ss R, Rd H, Saba N, Tk O, Kauh J, Dm S, Sy S, Strychor S, Tighiouart M, Egorin Mj FH, Fr K (2010) Phase 1 and pharmacokinetic study of Everolimus, a mammalian target of rapamycin inhibitor, in combination with docetaxel for recurrent/refractory nonsmall cell lung cancer. Cancer 116:3903–3909
O’reilly T, Pm M, Wartmann M, Lassota P, Brandt R, Ha L (2011) Evaluation of the Mtor inhibitor, Everolimus, in combination with cytotoxic antitumor agents using human tumor models in vitro and in vivo. Anticancer Drugs 22:58–78
Sandler A, Gray R, Mc P, Brahmer J, Schiller J, Dowlati A, Lilenbaum R, Johnson D (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355:2542–2550
Goldstraw P, Crowley J, Chansky K, Dj G, Pa G, Rami-Porta R, Pe P, Rusch V, Sobin L (2007) The IASLC lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2:706–714
Mg K, Pj H, Mr S, Feyer P, Clark-Snow R, Jm K, Gr M, Lw C, Mj C, Rj G, Sm G (2006) American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol 24:2932–2947
Herrstedt J, Ms A, Roila F, Vv K (2005) ESMO minimum clinical recommendations for prophylaxis of chemotherapy-induced nausea and vomiting (Nv). Ann Oncol 16(Suppl 1):I77–I79
Therasse P, Sg A, Ea E, Wanders J, Rs K, Rubinstein L, Verweij J, Van Glabbeke M, At VO, Mc C, Sg G (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Tabernero J, Rojo F, Calvo E, Burris H, Judson I, Hazell K, Martinelli E, Cajal S, Jones S, Vidal L, Shand N, Macarulla T, Fj R, Dimitrijevic S, Zoellner U, Tang P, Stumm M, Ha L, Lebwohl D, Baselga J (2008) Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol 26:1603–1610
O’donnell A, Faivre S, Ha B, Iii RD, Papadimitrakopoulou V, Shand N, Ha L, Hazell K, Zoellner U, Jm K, Brock C, Jones S, Raymond E, Judson I (2008) Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol 26:1588–1595
Xu B, Wu Y, Shen L, Ye D, Jappe A, Cherfi A, Wang H, Yuan R (2011) Two dose-level confirmatory study of the pharmacokinetics and tolerability of everolimus in Chinese patients with advanced solid tumors. J Hematol Oncol 4:3
Okamoto I, Doi T, Ohtsu A, Miyazaki M, Tsuya A, Kurei K, Kobayashi K, Nakagawa K (2010) Phase I clinical and pharmacokinetic study of Rad001 (Everolimus) administered daily to Japanese patients with advanced solid tumors. Jpn J Clin Oncol 40:17–23
Dt M, Gj R, Cg A, Je G, Rt H, Mg K, Lm K, Pao W, Pizzo B, Na R, Va M (2007) Phase 1 trial of everolimus and gefitinib in patients with advanced nonsmall-cell lung cancer. Cancer 110:599–605
Vansteenkiste J, Solomon B, Boyer M, Wolf J, Miller N, Sl D, Pylvaenaeinen I, Petrovic K, Dimitrijevic S, Anrys B, Laack E (2011) Everolimus in combination with pemetrexed in patients with advanced non-small cell lung cancer previously treated with chemotherapy: a phase I study using a novel, adaptive Bayesian dose-escalation model. J Thorac Oncol 6:2120–2129
Al H, Ej S, Vk W, Sun J, Cj R, Am L, Fong L, Dr B, Je R (2011) A phase 1 study of everolimus and sorafenib for metastatic clear cell renal cell carcinoma. Cancer 117:4194–4200
Ke B, Wp P, Younis I, He U, Ma M, Gc B, Sy Z, Jp G, Jj L, Truax R, Kl M, La H, Mm O’n, Broadwater G, Hi H, Jc B (2011) A Phase I study of bevacizumab (B) in combination with everolimus (E) and erlotinib (E) in advanced cancer (Bee). Cancer Chemother Pharmacol 67:465–474
Jc Y, Shah M, Ito T, Cl B, Em W, Van Cutsem E, Tj H, Okusaka T, Capdevila J, Eg DV, Tomassetti P, Me P, Hoosen S, Haas T, Lincy J, Lebwohl D, Oberg K (2011) Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 364:514–523
Acknowledgments
This study was supported by Novartis Pharmaceuticals Corporation. Editorial assistance in the preparation of this manuscript was provided by Melanie Leiby, PhD, of ApotheCom (Yardley, PA, USA) and was funded by Novartis Pharmaceuticals Corporation.
Conflict of interest
Jeffrey De Leo, Sven Gogov, Valentine Jehl, and Shweta Urva are employees of Novartis Pharmaceuticals. Sven Gogov and Shweta Urva hold stock in Novartis Pharmaceuticals.
Wilfried Eberhardt has received support for travel to meetings for the study or other purpose and provision of writing assistance, medicines, equipment, or administrative support from Novartis Pharmaceuticals; served as a consultant for Novartis Pharmaceuticals; received payment for lectures including service on speakers bureaus from Novartis Pharmaceuticals; and payment for development of educational presentations from Novartis Pharmaceuticals.
Joan Schiller has received consulting fees or honorarium from Novartis Pharmaceuticals.
Michael P. Brown has received grant funding from Novartis and served as an advisory board member for Novartis.
Michael Thomas has received grants, consulting fees or honorarium, support for travel to meetings, and fees for participation in other review activities from Novartis Pharmaceuticals.
Glenn Mills and Vassiliki Papadimitrakopoulou have grant funding from Novartis Pharmaceuticals.
Paul Mitchell declares no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eberhardt, W.E.E., Mitchell, P., Schiller, J.H. et al. Feasibility of adding everolimus to carboplatin and paclitaxel, with or without bevacizumab, for treatment-naive, advanced non–small cell lung cancer. Invest New Drugs 32, 123–134 (2014). https://doi.org/10.1007/s10637-013-9958-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-013-9958-3