Summary
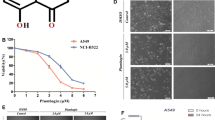
Caffeic acid phenethyl ester (CAPE) is a phenolic compound initially identified in bee glue. CAPE is reported to exhibit antitumor activity in many cancer models. However, the effect of CAPE on multiple myeloma (MM) is not well studied. We investigated the anti-myeloma effect of CAPE, and the data showed that CAPE inhibited the growth of human MM cells in a dose (1 ~ 30 μM) and time (24 ~72 h) dependent manner without altering the viability of normal human peripheral blood B cells. Stress and toxicity pathway analysis demonstrated that CAPE, in a dose- and time-related fashion, induced the expression of apoptotic and oxidative stress-response genes including growth arrest and DNA-damage inducible, alpha and gamma (GADD45A and GADD45G) and heme oxygenase-1. Apoptosis of MM cells by CAPE was further confirmed through flow cytometric analysis with up to 50% apoptotic cells induced by 50 μM CAPE within 24 h. Western blot analysis revealed the CAPE-induced activation of apoptosis executioner enzyme caspase-3, and corresponding cleavage of its downstream target poly(ADP-ribose)polymerase (PARP). The oxidative stress caused by CAPE cytotoxicity in MM cells was evaluated through measurement of reactive oxygen species (ROS) level, antioxidant intervention and glutathione depletion. The intracellular ROS level was not elevated by CAPE, but the pretreatment of antioxidant (N-acetyl cysteine) and glutathione synthesis inhibitor (buthionine sulfoximine) suggested that CAPE may cause oxidative stress by decrease of intracellular antioxidant level rather than over production of ROS. These data suggest that CAPE promotes apoptosis through oxidative stress in human multiple myeloma cells.





Similar content being viewed by others
References
Palumbo A, Anderson K (2011) Multiple myeloma. N Engl J Med 364(11):1046–1060. https://doi.org/10.1056/NEJMra1011442
Fairfax KA, Kallies A, Nutt SL, Tarlinton DM (2008) Plasma cell development: from B-cell subsets to long-term survival niches. Semin Immunol 20(1):49–58. https://doi.org/10.1016/j.smim.2007.12.002
Bakkus MH, Heirman C, Van Riet I, Van Camp B, Thielemans K (1992) Evidence that multiple myeloma Ig heavy chain VDJ genes contain somatic mutations but show no intraclonal variation. Blood 80(9):2326–2335
Fonseca R, Barlogie B, Bataille R, Bastard C, Bergsagel PL, Chesi M, Davies FE, Drach J, Greipp PR, Kirsch IR, Kuehl WM, Hernandez JM, Minvielle S, Pilarski LM, Shaughnessy JD Jr, Stewart AK, Avet-Loiseau H (2004) Genetics and cytogenetics of multiple myeloma: a workshop report. Cancer Res 64(4):1546–1558
Chauhan D, Uchiyama H, Urashima M, Yamamoto K, Anderson KC (1995) Regulation of interleukin 6 in multiple myeloma and bone marrow stromal cells. Stem Cells 13(Suppl 2):35–39
Podar K, Tai YT, Davies FE, Lentzsch S, Sattler M, Hideshima T, Lin BK, Gupta D, Shima Y, Chauhan D, Mitsiades C, Raje N, Richardson P, Anderson KC (2001) Vascular endothelial growth factor triggers signaling cascades mediating multiple myeloma cell growth and migration. Blood 98(2):428–435
Mitsiades CS, Mitsiades NS, Munshi NC, Richardson PG, Anderson KC (2006) The role of the bone microenvironment in the pathophysiology and therapeutic management of multiple myeloma: interplay of growth factors, their receptors and stromal interactions. Eur J Cancer 42(11):1564–1573. https://doi.org/10.1016/j.ejca.2005.12.025
Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC (2007) Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer 7(8):585–598. https://doi.org/10.1038/nrc2189
Hazlehurst LA, Dalton WS (2001) Mechanisms associated with cell adhesion mediated drug resistance (CAM-DR) in hematopoietic malignancies. Cancer Metastasis Rev 20(1–2):43–50
Dalton WS (2003) The tumor microenvironment: focus on myeloma. Cancer Treat Rev 29(Suppl 1):11–19
Younes H, Leleu X, Hatjiharissi E, Moreau AS, Hideshima T, Richardson P, Anderson KC, Ghobrial IM (2007) Targeting the phosphatidylinositol 3-kinase pathway in multiple myeloma. Clin Cancer Res 13(13):3771–3775. https://doi.org/10.1158/1078-0432.CCR-06-2921
Ishikawa H, Tsuyama N, Abroun S, Liu S, Li FJ, Otsuyama K, Zheng X, Kawano MM (2003) Interleukin-6, CD45 and the src-kinases in myeloma cell proliferation. Leuk Lymphoma 44(9):1477–1481. https://doi.org/10.3109/10428190309178767
Li ZW, Chen H, Campbell RA, Bonavida B, Berenson JR (2008) NF-kappaB in the pathogenesis and treatment of multiple myeloma. Curr Opin Hematol 15(4):391–399. https://doi.org/10.1097/MOH.0b013e328302c7f4
Hideshima T, Bergsagel PL, Kuehl WM, Anderson KC (2004) Advances in biology of multiple myeloma: clinical applications. Blood 104(3):607–618. https://doi.org/10.1182/blood-2004-01-0037
Gentile M, Recchia AG, Mazzone C, Lucia E, Vigna E, Morabito F (2013) Perspectives in the treatment of multiple myeloma. Expert Opin Biol Ther 13(Suppl 1):S1–S22. https://doi.org/10.1517/14712598.2013.799132
Gullett NP, Ruhul Amin AR, Bayraktar S, Pezzuto JM, Shin DM, Khuri FR, Aggarwal BB, Surh YJ, Kucuk O (2010) Cancer prevention with natural compounds. Semin Oncol 37(3):258–281. https://doi.org/10.1053/j.seminoncol.2010.06.014
Patel S (2016) Emerging adjuvant therapy for Cancer: Propolis and its constituents. J Diet Suppl 13(3):245–268. https://doi.org/10.3109/19390211.2015.1008614
Son S, Lewis BA (2002) Free radical scavenging and antioxidative activity of caffeic acid amide and ester analogues: structure-activity relationship. J Agric Food Chem 50(3):468–472
Toyoda T, Tsukamoto T, Takasu S, Shi L, Hirano N, Ban H, Kumagai T, Tatematsu M (2009) Anti-inflammatory effects of caffeic acid phenethyl ester (CAPE), a nuclear factor-kappaB inhibitor, on helicobacter pylori-induced gastritis in Mongolian gerbils. Int J Cancer 125(8):1786–1795. https://doi.org/10.1002/ijc.24586
Tseng TH, Lee YJ (2006) Evaluation of natural and synthetic compounds from east Asiatic folk medicinal plants on the mediation of cancer. Anti Cancer Agents Med Chem 6(4):347–365
Natarajan K, Singh S, Burke TR Jr, Grunberger D, Aggarwal BB (1996) Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc Natl Acad Sci U S A 93(17):9090–9095
Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB (2010) Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 49(11):1603–1616. https://doi.org/10.1016/j.freeradbiomed.2010.09.006
Wu J, Omene C, Karkoszka J, Bosland M, Eckard J, Klein CB, Frenkel K (2011) Caffeic acid phenethyl ester (CAPE), derived from a honeybee product propolis, exhibits a diversity of anti-tumor effects in pre-clinical models of human breast cancer. Cancer Lett 308(1):43–53. https://doi.org/10.1016/j.canlet.2011.04.012
Lin HP, Jiang SS, Chuu CP (2012) Caffeic acid phenethyl ester causes p21 induction, Akt signaling reduction, and growth inhibition in PC-3 human prostate cancer cells. PLoS One 7(2):e31286. https://doi.org/10.1371/journal.pone.0031286
Akyol S, Ozturk G, Ginis Z, Armutcu F, Yigitoglu MR, Akyol O (2013) In vivo and in vitro antineoplastic actions of caffeic acid phenethyl ester (CAPE): therapeutic perspectives. Nutr Cancer 65(4):515–526. https://doi.org/10.1080/01635581.2013.776693
Wang X, Stavchansky S, Zhao B, Bynum JA, Kerwin SM, Bowman PD (2008) Cytoprotection of human endothelial cells from menadione cytotoxicity by caffeic acid phenethyl ester: the role of heme oxygenase-1. Eur J Pharmacol 591(1–3):28–35. https://doi.org/10.1016/j.ejphar.2008.06.017
Zaman S, Wang R, Gandhi V (2014) Targeting the apoptosis pathway in hematologic malignancies. Leuk Lymphoma 55(9):1980–1992. https://doi.org/10.3109/10428194.2013.855307
Walker RE, Lawson MA, Buckle CH, Snowden JA, Chantry AD (2014) Myeloma bone disease: pathogenesis, current treatments and future targets. Br Med Bull 111(1):117–138. https://doi.org/10.1093/bmb/ldu016
Bynum JA, Wang X, Stavchansky SA, Bowman PD (2017) Time course expression analysis of 1[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole induction of Cytoprotection in human endothelial cells. Gene Regul Syst Bio 11: https://doi.org/10.1177/1177625017701106
Nicholson JK, Connelly J, Lindon JC, Holmes E (2002) Metabonomics: a platform for studying drug toxicity and gene function. Nat Rev Drug Discov 1(2):153–161. https://doi.org/10.1038/nrd728
Orrenius S, Nicotera P, Zhivotovsky B (2011) Cell death mechanisms and their implications in toxicology. Toxicol Sci 119(1):3–19. https://doi.org/10.1093/toxsci/kfq268
Beauregard AP, Harquail J, Lassalle-Claux G, Belbraouet M, Jean-Francois J, Touaibia M, Robichaud GA (2015) CAPE analogs induce growth arrest and apoptosis in breast Cancer cells. Molecules 20(7):12576–12589. https://doi.org/10.3390/molecules200712576
Ozturk G, Ginis Z, Akyol S, Erden G, Gurel A, Akyol O (2012) The anticancer mechanism of caffeic acid phenethyl ester (CAPE): review of melanomas, lung and prostate cancers. Eur Rev Med Pharmacol Sci 16(15):2064–2068
Nowsheen S, Yang ES (2012) The intersection between DNA damage response and cell death pathways. Exp Oncol 34(3):243–254
Ozben T (2007) Oxidative stress and apoptosis: impact on cancer therapy. J Pharm Sci 96(9):2181–2196. https://doi.org/10.1002/jps.20874
Kirshner JR, He S, Balasubramanyam V, Kepros J, Yang CY, Zhang M, Du Z, Barsoum J, Bertin J (2008) Elesclomol induces cancer cell apoptosis through oxidative stress. Mol Cancer Ther 7(8):2319–2327. https://doi.org/10.1158/1535-7163.MCT-08-0298
Leon-Gonzalez AJ, Auger C, Schini-Kerth VB (2015) Pro-oxidant activity of polyphenols and its implication on cancer chemoprevention and chemotherapy. Biochem Pharmacol 98(3):371–380. https://doi.org/10.1016/j.bcp.2015.07.017
Rahal A, Kumar A, Singh V, Yadav B, Tiwari R, Chakraborty S, Dhama K (2014) Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed Res Int 2014:761264–761219. https://doi.org/10.1155/2014/761264
Funding
This work was supported by the American Association of Colleges of Pharmacy (AACP) New Investigator Award and The Center for Chronic Disorders of Aging (CCDA) funding from Philadelphia College of Osteopathic Medicine to XW. It was partly supported by AHA (11SDG5710004) and NIAID (AI128254-01A1) to RS.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: XW. Performed the experiments: EM, HP, ML.YB, and MK. Analyzed the data: EM, HP, ML, RS, and XW. Critically reviewed the paper: RS. Wrote the paper: XW.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Marin, E.H., Paek, H., Li, M. et al. Caffeic acid phenethyl ester exerts apoptotic and oxidative stress on human multiple myeloma cells. Invest New Drugs 37, 837–848 (2019). https://doi.org/10.1007/s10637-018-0701-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-018-0701-y