Abstract
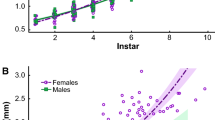
In sexual species, phenotypic divergence between males and females, or sexual dimorphism, is often the source of the most staggering examples of phenotypic variation in nature. Theory suggests that exaggerated sexual traits should drive sex-specific nutritional demands. Advances in spectrometry enable rapid quantification of the elements that make up individuals and traits, which can be used to assess patterns of intraspecific variation and the contribution of nutritionally-demanding sexual traits to these patterns. We measured dimorphism in the whole body stoichiometry of Hyalella amphipods and examined whether nutritional demands of exaggerated sexual traits differ from those of similar traits not under sexual selection. We found striking sexual dimorphism in multivariate whole body elemental composition (i.e., the ionome), including elements important for organismal growth and performance. In males, the exaggerated, sexually-selected claw-like appendage (posterior gnathopod) differed significantly in mass-specific stoichiometry from a similarly sized and serially homologous non-sexual trait (fifth pereopod), indicating that there are fundamental differences in the construction of sexual traits in relation to similar traits that are not under sexual selection. While sexually selected traits do differ from non-sexual traits in their ionomes, we found that possessing an exaggerated trait does not change organismal stoichiometry, indicating that trait exaggeration may not be directly driving ionomic sexual dimorphism. Finally, we found that larger traits are not comparatively larger resource sinks for any element, suggesting that the possession of larger traits is not a function of greater allocation of resources. Together, we discovered substantial sexual dimorphism at the lowest level of organization, chemical elements. Such information illuminates predictions about dimorphisms in foraging behavior, nutritional physiology, and sex-specific selection on the underlying loci. High throughput, multidimensional data on sexual divergence in stoichiometric composition is a powerful tool in understanding the evolutionary ecology of sexual dimorphisms.



Similar content being viewed by others
References
Ahearn GA, Mandal PK, Mandal A (2004) Calcium regulation in crustaceans during the molt cycle: a review and update. Comp Biochem Physiol Part A 137:247–257
Andersson MB (1994) Sexual selection, 1st edn. Princeton University Press, Princeton
Back JA, King RS (2013) Sex and size matter: ontogenetic patterns of nutrient content of aquatic insects. Freshw Sci 32:837–848
Bertram SM, Bowen M, Kyle M, Schade JD (2008) Extensive natural intraspecific variation in stoichiometric (C:N:P) composition in two terrestrial insect species. J Insect Sci 8:1–7
Bertram SM, Whattam EM, Visanuvimol L et al (2009) Phosphorus availability influences cricket mate attraction displays. Anim Behav 77:525–530
Bonduriansky R (2007) Sexual selection and allometry: a critical reappraisal of the evidence and ideas. Evolution 61:838–849
Bonduriansky R, Maklakov A, Zajitschek F, Brooks R (2008) Sexual selection, sexual conflict and the evolution of ageing and life span. Funct Ecol 22:443–453
Brosnan J, Brosnan M (2006) The sulfur-containing amino acids: an overview. J Nutr 136:16365–16405
Connallon T, Cox RM, Calsbeek R (2010) Fitness consequences of sex-specific selection. Evolution 64:1671–1682
Cothran RD, Jeyasingh PD (2010) Condition dependence of a sexually selected trait in a crustacean species complex: importance of the ecological context. Evolution 64:2535–2546
Cothran RD, Kuzmic A, Wellborn GA, Relyea RA (2010) Phenotypic manipulation provides insights into the function of a sexually selected trait in a freshwater crustacean species complex. Anim Behav 80:543–549
Cothran RD, Stiff AR, Jeyasingh PD, Relyea RA (2012) Eutrophication and predation risk interact to affect sexual trait expression and mating success. Evolution 66:708–719
Cotton S, Fowler K, Pomiankowski A (2004) Do sexual ornaments demonstrate heightened condition-dependent expression as predicted by the handicap hypothesis? Proc R Soc Lond B Biol Sci 271:771–783
Cowan RL, Hartsook EW, Whelan JB (1968) Calcium–strontium metabolism in white tailed deer as related to age and antler growth. Exp Biol Med 129:733–737
Cullen JT, Sherrell RM (2005) Effects of dissolved carbon dioxide, zinc, and manganese on the cadmium to phosphorus ratio in natural phytoplankton assemblages. Limnol Oceanogr 50:1193–1204
Dudley R, Kaspari M, Yanoviak SP (2012) Lust for salt in the western Amazon. Biotropica 44:6–9
Edwards TD, Cowell BC (1992) Population dynamics and secondary production of Hyalella azteca (Amphipoda) in Typha stands of a subtropical Florida lake. J N Am Benthol Soc 11:69–79
Ellegren H, Parsch J (2007) The evolution of sex-biased genes and sex-biased gene expression. Nat Rev Genet 8:689–698
El-Sabaawi RW, Zandonà E, Kohler TJ et al (2012) Widespread intraspecific organismal stoichiometry among populations of the Trinidadian guppy. Funct Ecol 26:666–676
El-Sabaawi RW, Travis J, Zandonà E et al (2014) Intraspecific variability modulates interspecific variability in animal organismal stoichiometry. Ecol Evol 4:1505–1515
Elser JJ, Dobberfuhl DR, MacKay NA (1996) Organism size, life history, and N:P stoichiometry. Bioscience 46:674–684
Emlen DJ (2001) Costs and the diversification of exaggerated animal structures. Science 291:1534–1536
Faerovig PJ, Hessen DO (2003) Allocation strategies in crustacean stoichiometry: the potential role of phosphorus in the limitation of reproduction. Freshw Biol 48:1782–1792
Fagan WF, Siemann E, Mitter C et al (2002) Nitrogen in insects: implications for trophic complexity and species diversification. Am Nat 160:784–802
Fairbairn DJ, Roff DA (2006) The quantitative genetics of sexual dimorphism: assessing the importance of sex-linkage. Heredity 97:319–328. doi:10.1038/sj.hdy.6800895
Frausto da Silva JJR, Williams RJP (1991) The biological chemistry of the elements: the inorganic chemistry of life. Oxford University Press, Oxford
Giardini J-L, Yan ND, Heyland A (2015) Consequences of calcium decline on the embryogenesis and life history of Daphnia magna. J Exp Biol 218:2005–2014
Gibbons WN, Mackie GL (1991) The relationship between environmental variables and demographic patterns of Hyalella azteca (Crustacea: Amphipoda). J N Am Benthol Soc 10:444–454
González AL, Fariña JM, Kay AD et al (2011) Exploring patterns and mechanisms of interspecific and intraspecific variation in body elemental composition of desert consumers. Oikos 120:1247–1255
Goos JM, Cothran RD, Jeyasingh PD (2014a) Subtle variation in phosphorus availability influences mating biology in Hyalella (Amphipoda: Hyalellidae) amphipods. Biol J Linn Soc 111:878–888
Goos JM, French BJ, Relyea RA et al (2014b) Sex-specific plasticity in body phosphorus content of Hyalella amphipods. Hydrobiologia 722:93–102
Goos JM, Cothran RD, Jeyasingh PD (2016) Sex-specific nutrient use and preferential allocation of resources to a sexually selected trait in Hyalella amphipods. J Exp Biol 219:649–657
Gunatilaka A (1981) Biogeochemistry of strontium. In: Skoryna SC (ed) Handbook of stable strontium, 1st edn. Plenum Press, New York, pp 19–46
Hedrick A (2005) Environmental condition-dependent effects on a heritable, preferred male trait. Anim Behav 70:1121–1124
Hessen DO, Alstad NEW, Skardal L (2000) Calcium limitation in Daphnia magna. J Plankton Res 22:553–568
Houle D, Govindaraju DR, Omholt S (2010) Phenomics: the next challenge. Nat Rev Genet 11:855–866
Jackson DA (1993) Stopping rules in principal components analysis: a comparison of heuristical and statistical approaches. Ecology 74:2204–2214
Karimi R, Folt CL (2006) Beyond macronutrients: element variability and multielement stoichiometry in freshwater invertebrates. Ecol Lett 9:1273–1283
Kay AD, Ashton IW, Gorokhova E et al (2005) Toward a stoichiometric framework for evolutionary biology. Oikos 109:6–17
Kilham S, Kreeger D, Lynn S, Goulden C (1998) COMBO: a defined freshwater culture medium for algae and zooplankton. Hydrobiologia 377:147–159
Kotiaho JS (2001) Costs of sexual traits: a mismatch between theoretical considerations and empirical evidence. Biol Rev 76:365–376
Lucu C (1990) Ionic regulatory mechanisms in crustacean gill epithelia. Comp Biochem Physiol Part A Physiol 97:297–306
Main TM, Dobberfuhl DR, Elser JJ (1997) N:P stoichiometry and ontogeny of crustacean zooplankton: a test of the growth rate hypothesis. Limnol Oceanogr 42:1474–1478
Markow TA, Dobberfuhl DR, Breitmeyer CM et al (1999) Elemental stoichiometry of Drosophila and their hosts. Funct Ecol 13:78–84
Markow TA, Coppola A, Watts TD (2001) How Drosophila males make eggs: it’s elemental. Proc R Soc Lond B Biol Sci 268:1527–1532
Matsumura M (1981) Strontium as the substitute for calcium in the excitation–contraction coupling of crayfish muscle fibers. In: Skoryna SC (ed) Handbook of stable strontium, 1st edn. Plenum Press, New York, pp 309–319
Morehouse NI, Nakazawa T, Booher CM et al (2010) Sex in a material world: why the study of sexual reproduction and sex-specific traits should become more nutritionally-explicit. Oikos 119:766–778
Morehouse RL, Dzialowski AR, Jeyasingh PD (2012) Impacts of excessive dietary phosphorus on zebra mussels. Hydrobiologia 707:73–80
Morritt D (1989) Ionic regulation in littoral and terrestrial amphipods (Crustacea: Amphipoda: Talitridae). J Exp Mar Biol Ecol 132:53–67
Nakazawa T (2011) The ontogenetic stoichiometric bottleneck stabilizes herbivore–autotroph dynamics. Ecol Res 26:209–216
Pilati A, Vanni MJ (2007) Ontogeny, diet shifts, and nutrient stoichiometry in fish. Oikos 116:1663–1674
Prater C, Wagner ND, Frost PC (2016) Effects of calcium and phosphorus limitation on the nutritional ecophysiology of Daphnia. Limnol Oceanogr 61:268–278
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
R Core Team (2016) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Rowe L, Houle D (1996) The lek paradox and the capture of genetic variance by condition dependent traits. Proc R Soc Lond B Biol Sci 263:1415–1421
Schade J, Kyle M, Hobbie S et al (2003) Stoichiometric tracking of soil nutrients by a desert insect herbivore. Ecol Lett 6:96–101
Schou M (1957) Biology and pharmacology of the lithium ion. Pharmacol Rev 9:17–58
Schrauzer GN, Shrestha KP, Flores-Arce MF (1992) Lithium in scalp hair of adults, students, and violent criminals: effects of supplementation and evidence for interactions of lithium with vitamin B12 and with other trace elements. Biol Trace Elem Res 34:161–176
Small GE, Pringle CM (2010) Deviation from strict homeostasis across multiple trophic levels in an invertebrate consumer assemblage exposed to high chronic phosphorus enrichment in a neotropical stream. Oecologia 162:581–590
Tobler M, Alba DM, Arias-Rodríguez L, Jeyasingh PD (2016) Using replicated evolution in extremophile fish to understand diversification in elemental composition and nutrient excretion. Freshw Biol 61:158–171
Visanuvimol L, Bertram SM (2010) Dietary phosphorus availability influences female cricket lifetime reproductive effort. Ecol Entomol 35:386–395
Wellborn GA (1995) Determinants of reproductive success in freshwater amphipod species that experience different mortality regimes. Anim Behav 50:353–363
Wellborn GA (2000) Selection on a sexually dimorphic trait in ecotypes within the Hyalella azteca species complex (Amphipoda: Hyalellidae). Am Midl Nat 143:212–225
Wellborn GA, Bartholf SE (2005) Ecological context and the importance of body and gnathopod size for pairing success in two amphipod ecomorphs. Oecologia 143:308–316
Wellborn GA, Broughton RE (2008) Diversification on an ecologically constrained adaptive landscape. Mol Ecol 17:2927–2936
Woods H, Fagan W, Elser J, Harrison J (2004) Allometric and phylogenetic variation in insect phosphorus content. Funct Ecol 18:103–109
Wright SJ, Yavitt JB, Wurzburger N et al (2011) Potassium, phosphorus, or nitrogen limit root allocation, tree growth, or litter production in a lowland tropical forest. Ecology 92:1616–1625
Zahavi A (1975) Mate selection—a selection for a handicap. J Theor Biol 53:205–214
Zehmer JK, Mahon SA, Capelli GM (2002) Calcium as a limiting factor in the distribution of the amphipod Gammarus pseudolimnaeus. Am Midl Nat 148:350–362
Acknowledgements
We thank P. Roy Chowdhury, J.B. Belden, and S.M. Morrison for their help in developing the ICP-OES protocol, and the three anonymous reviewers for their constructive comments that improved this manuscript. This work was supported in part by National Science Foundation Grant No. 0924401 and the Distinguished Graduate Fellowship from the Graduate College at Oklahoma State University. This research is part of the Ph.D. dissertation of JMG at Oklahoma State University. JMG thanks PDJ, M. Tobler, J.A. Steets, and M. Papeş for serving on the doctoral committee.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Goos, J.M., Cothran, R.D. & Jeyasingh, P.D. Within-population variation in the chemistry of life: the stoichiometry of sexual dimorphism in multiple dimensions. Evol Ecol 31, 635–651 (2017). https://doi.org/10.1007/s10682-017-9900-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-017-9900-9