Abstract
By analysing a contemporary criticism to the so called “mathematical chemistry”, we discuss what we understand by mathematizing chemistry and its implications. We then pass to ponder on some positions on the subject by considering the cases of Laszlo, Venel and Diderot, opponents to the idea of mathematization of chemistry. In contrast, we analyse some scholars’ ideas on the fruitful relationship between mathematics and chemistry; here Dirac and Brown are considered. Finally, we mention that the mathematical–chemistry relationship should be considered beyond the mere aspect of whether chemistry is or not able to be mathematized. This discussion is based upon opinions by Kant and Comte, the first one having two positions on chemistry based upon mathematics and the latter mooting the idea of doing chemistry with mathematical spirit.
Similar content being viewed by others
Notes
A great disseminator and editor of mathematical chemistry documents is Dennis H. Rouvray. The three journals are MATCH Communications in Mathematical and in Computer Chemistry, Journal of Mathematical Chemistry and Iranian Journal of Mathematical Chemistry.
The website of the International Academy of Mathematical Chemistry is http://www.iamc-online.org/index.htm.
Another example is given by Latour (1987) when explaining that once a town is put on a map, “a list of two-figure readings (longitudes and latitudes) […] is now transformed into points on a curved surface figuring the earth”. Then the question is “how to go from the curved to the flat surface without further deformation? […] how to project the sphere on a surface?” Latour mentions that this important question for geographers, with implications for navigators and in the end for the economies, is an instance of how “abstract” geometry has social links.
As a token of this, one can recall the Lucasian chair of mathematics at Cambridge.
In the Encyclopédie ou Dictionnaire raisonné des sciences, des arts et des métiers, by Diderot and D’Alembert, it is seen that chemistry (Chimie) is classified as “particular physics” (Physique particuliere) along with zoology (that includes medicine), astronomic physics (that includes astrology), meteorology, cosmology, botany, and mineralogy. Mathematics gathers arithmetic, geometry, mechanics, geometric astronomy, optics, acoustics, pneumatics, and conjecture art (that includes risk analysis). Also, in a subtle level of the mathematical classification, physicomathematics are included.
Thoughts on the interpretation of nature.
Brown pondered on the relationship between chemistry and mathematics, for he was active in it. In 1865, he published the influential paper “On the theory of isomeric compounds” (Brown 1865), where, according to Klein, in a forthcoming paper on the relationship between mathematics and chemistry, a correspondence between molecules and isomorphic classes of coloured graphs is established: four bonds to each C-colour, one bond to each H-colour, and two bonds to each O-colour. “He does not use these words”, Klein continues, “as they were not yet in use, but this would be the modern (mathematical) way to describe what Brown proposed”.
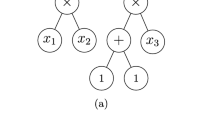
Trinajstić (1997) makes reference to an 1866/1867 paper by Brown where the Scottish chemist treats chemical substances as “operands” and chemical processes as “operators”. According to Brown, \( \phi \cdot S \) represents the substance obtained from substance S by the process \( \phi \). Different processes \( \phi \), \( \chi \), ψ, etc. acting independently on S are denoted by:
$$ \left. {\begin{array}{*{20}c} \phi \hfill \\ \chi \hfill \\ \psi \hfill \\ {{\text{etc}}.} \hfill \\ \end{array} } \right] \cdot S $$When a concatenation of processes takes place on S, this is represented by \( \phi \cdot \chi \cdot \psi \cdot S \). He mentions that commutative law does not apply to operators and also defines \( \phi^{ - 1} \) as the operator satisfying \( \phi \cdot (\phi^{ - 1} \cdot S) = S. \)
Kant quotes refer to the Akademie Ausgabe and are cited giving the volume and the page number (Kant 1900–2000).
“It mus be owned that, with regard to doctrine, the connection of Chemistry with the first two sciences [astronomy and mathematics] is neither extensive nor very important” (Comte 1893). Even if that relation is quite difficult to Comte, he thinks chemistry is more akin to astronomy than to mathematics: “when the time shall come for the development of concrete chemistry,—that is, the methodical application of chemical knowledge to the natural history of the globe,—astronomical considerations will no doubt enter in where now there seems no point of contact between the two sciences” (Comte 1893).
In this respect he also says: “It is only by having witnessed the purification in the anterior sciences [mathematics, astronomy and then physics] that chemists could realize it in their own” (Comte 1893).
References
Balaban, A.T.: Reflections about mathematical chemistry. Found. Chem. 7, 289–306 (2005)
Bourdeau, M.: Auguste Comte. In: Zalta, E.N. (ed.) The Stanford encyclopedia of philosophy (summer 2011 edition). URL: http://plato.stanford.edu/archives/sum2011/entries/comte/ (2011)
Brown, A.C.: On the theory of isomeric compounds. J. Chem. Soc. 18, 230–245 (1865) (this paper is a reprint, the original was published in Brown, A.C.: On the theory of isomeric compounds. T. Roy. Soc. Edin. 23, 707 (1864))
Brown, A.C.: Address to the chemical section. Report of the Forty-Fourth Meeting of the British Association for the Advancement of Science. http://archive.org/stream/reportofbritisha75brit#page/n5/mode/2up (1875). Accessed 20 June 2012
Comte, A.: The Positive Philosophy of Auguste Comte (ed. and trans. by H. Martineau). Kegan Paul, London (1893)
Dirac, P.A.M.: Quantum mechanics of many-electron systems. Proc. R. Soc. A. 123, 714–733 (1929)
Förster, E. (trans. and ed.): Kant’s Opus Postumum. Cambridge University Press, Cambridge (1993)
Gillispie, C.C.: The Edge of Objectivity. Princeton University Press, Princeton (1960)
Kant, I.: Akademieausgabe von Immanuel Kants Gesammelten Werken Bände und Verknüpfungen zu den Inhaltsverzeichnissen. Korpora.org. http://www.korpora.org/kant/verzeichnisse-gesamt.html (1900–2000). Accessed 20 June 2012
Klein, D.J.: Personal communication
Klotz, I.M., Rosenberg, R.M.: Chemical thermodynamics, basic concepts and methods. Wiley, Hoboken (2008)
Laszlo, P.: Circulation of concepts. Found. Chem. 1, 225–238 (1999)
Latour, B.: Science in action. Harvard University Press, Cambridge (1987)
Leal, W., Restrepo, G., Bernal, A.: A network study of chemical elements: from binary compounds to chemical trends. MATCH Commun. Math. Comput. Chem. 68, 411–436 (2012)
Liegener, C., Del Re, G.: Chemistry vs. physics, the reduction myth, and the unity of science. J. Gen. Philos. Sci. 18, 165–174 (1987)
Lombardi, O., Labarca, M.: The ontological autonomy of the chemical world. Found. Chem. 7, 125–148 (2005)
Mainzer, K.: Computational and mathematical models in chemistry, epistemic foundations and new perspectives of research. In: Janich, P., Psarros, N. (eds.) The Autonomy of Chemistry, 3rd Erlenmeyer–Colloquy for the Philosophy of Chemistry, pp. 33–50. Königshausen & Neumann, Würzburg (1998)
Mirkin, B.: Mathematical classification and clustering. Kluwer, Dordrecht (1996)
Restrepo, G., Villaveces, J.L.: Chemistry, a lingua philosophica. Found. Chem. 13, 233–249 (2011)
Restrepo, G., Villaveces, J.L.: Mathematical thinking in chemistry. HYLE 18, 3–22 (2012a)
Scerri, E.R.: The electronic configuration model, quantum mechanics and reduction. Brit. J. Philos. Sci. 42, 309–325 (1991)
Scerri, E.R.: Has chemistry been at least approximately reduced to quantum mechanics? Proc. Biennial Meet. Philos. Sci. Assoc. 1, 160–170 (1994)
Scerri, E.: The ambiguity of reduction. HYLE 13, 67–81 (2007)
Schummer, J.: The chemical core of chemistry I, a conceptual approach. HYLE 4, 129–162 (1998)
Smith, D.E.: History of mathematics. General survey of the history of elementary mathematics, vol. I. Dover, New York (1958)
Trinajstić, N.: Mathematics and chemistry—the unlikely partners. In: Rouvray, D.H. (ed.) Concepts in Chemistry, A Contemporary Challenge, pp. 17–39. Research Studies Press, Somerset (1997)
Trinajstić, N., Gutman, I.: Mathematical chemistry. Croat. Chem. Acta 75, 329–356 (2002)
Van Brakel, J.: Kant’s legacy for the philosophy of chemistry. In: Baird, D., Scerri, E. (eds.) Philosophy of Chemistry, pp. 69–91. Springer, Berlin (2006)
Weyl, H.: The mathematical way of thinking. Science 92, 437–446 (1940)
Acknowledgments
The author thanks J. L. Villaveces (Universidad de los Andes, Colombia), D. J. Klein (Texas A&M University at Galveston, USA) and Rom Harré (Georgetown University, USA and London School of Economics, UK) for their valuable comments, ideas, and suggestions, which have been included in this manuscript. The author also thanks J. Schummer (Germany) for his valuable comments to the first drafts of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Restrepo, G. To mathematize, or not to mathematize chemistry. Found Chem 15, 185–197 (2013). https://doi.org/10.1007/s10698-013-9183-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10698-013-9183-3