Abstract
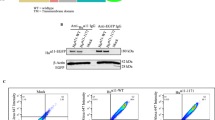
Integrins can function synergistically with syndecan-4 (SYND4) and bind to the fibronectin (FN) matrix, resulting in the regulation of tissue regeneration. This study aimed to explore the effects of TNF-α on the formation of FN/ITGβ1/SYND4 complex and the relative mechanism in NP cells. The expression of FN-ITG-SYND4 at the cellular level under TNF-α stimulation was detected by immunofluorescent staining, western blotting, and RT-PCR. ITGβ1 is a crucial component of ITG FN-induced FAK signaling, which was detected using dual mode. And, the involved signaling down stream pathways were also detected. FN is a preferred adhesion substrate for NP cells and that integrin β1 (ITGβ1) and SYND4 work synergistically during ECM engagement in a focal adhesion kinase (FAK)-dependent fashion. The PI3k/Akt pathway is obviously down-regulated, resulting in decreased adherence capacity and increased anoikis. TNF-α induction could weaken FAK activity and downstream levels of phospho-PI3K and Akt, resulting in decreased adherence capacity and increased apoptosis. Thus, TNF-α is essential for the formation of FN/ITGβ1/SYND4 complex in NP cells and further elucidates the inflammatory mechanism of NP cells degeneration.





Similar content being viewed by others
References
Adams, M.A., P. Dolan, and W.C. Hutton. 1986. The stages of disc degeneration as revealed by discograms. Journal of Bone and Joint Surgery. British Volume (London) 68 (1): 36–41.
Binch, A.L., I.M. Shapiro, and M.V. Risbud. 2016. Syndecan-4 in intervertebral disc and cartilage: Saint or synner? Matrix Biology 52 (54): 355–362.
Feng, H., M. Danfelter, B. Stromqvist, and D. Heinegard. 2006. Extracellular matrix in disc degeneration. The Journal of Bone and Joint Surgery. American Volume 88 (Suppl 2): 25–29.
Freemont, A.J. 2009. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology (Oxford) 48 (1): 5–10.
Frisch, S.M., K. Vuori, E. Ruoslahti, and P.Y. Chan-Hui. 1996. Control of adhesion-dependent cell survival by focal adhesion kinase. The Journal of Cell Biology 134 (3): 793–799.
Hoyland, J.A., C. Le Maitre, and A.J. Freemont. 2008. Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc. Rheumatology (Oxford) 47 (6): 809–814.
Kainulainen, V., H. Wang, C. Schick, and M. Bernfield. 1998. Syndecans, heparan sulfate proteoglycans, maintain the proteolytic balance of acute wound fluids. The Journal of Biological Chemistry 273 (19): 11563–11569.
Le Maitre, C.L., A.J. Freemont, and J.A. Hoyland. 2005. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Research & Therapy 7 (4): R732–R745.
Le Maitre, C.L., J.A. Hoyland, and A.J. Freemont. 2007a. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Research & Therapy 9 (4): R77.
Le Maitre, C.L., J.A. Hoyland, and A.J. Freemont. 2007b. Interleukin-1 receptor antagonist delivered directly and by gene therapy inhibits matrix degradation in the intact degenerate human intervertebral disc: An in situ zymographic and gene therapy study. Arthritis Research & Therapy 9 (4): R83.
Le Maitre, C.L., A. Pockert, D.J. Buttle, A.J. Freemont, and J.A. Hoyland. 2007c. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochemical Society Transactions 35 (Pt 4): 652–655.
Mostafavi-Pour, Z., J.A. Askari, S.J. Parkinson, P.J. Parker, T.T. Ng, and M.J. Humphries. 2003. Integrin-specific signaling pathways controlling focal adhesion formation and cell migration. The Journal of Cell Biology 161 (1): 155–167.
Mwale, F., P. Roughley, and J. Antoniou. 2004. Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: A requisite for tissue engineering of intervertebral disc. European Cells & Materials 8: 58–63 discussion 63-54.
Ouyang, Z.H., W.J. Wang, Y.G. Yan, B. Wang, and G.H. Lv. 2017. The PI3K/Akt pathway: A critical player in intervertebral disc degeneration. Oncotarget. 8: 57870–57881.
Shamji, M.F., L.A. Setton, W. Jarvis, S. So, J. Chen, L. Jing, R. Bullock, R.E. Isaacs, C. Brown, and W.J. Richardson. 2010. Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis and Rheumatism 62 (7): 1974–1982.
Subramanian, S.V., M.L. Fitzgerald, and M. Bernfield. 1997. Regulated shedding of syndecan-1 and -4 ectodomains by thrombin and growth factor receptor activation. The Journal of Biological Chemistry 272 (23): 14713–14720.
Tran, C.M., Z.R. Schoepflin, D.Z. Markova, C.K. Kepler, D.G. Anderson, I.M. Shapiro, and M.V. Risbud. 2014. CCN2 suppresses catabolic effects of interleukin-1beta through alpha5beta1 and alphaVbeta3 integrins in nucleus pulposus cells: Implications in intervertebral disc degeneration. The Journal of Biological Chemistry 289 (11): 7374–7387.
Wang, Z., R.J. Collighan, S.R. Gross, E.H. Danen, G. Orend, D. Telci, and M. Griffin. 2010. RGD-independent cell adhesion via a tissue transglutaminase-fibronectin matrix promotes fibronectin fibril deposition and requires syndecan-4/2 alpha5beta1 integrin co-signaling. The Journal of Biological Chemistry 285 (51): 40212–40229.
Wang, J., D. Markova, D.G. Anderson, Z. Zheng, I.M. Shapiro, and M.V. Risbud. 2011a. TNF-alpha and IL-1beta promote a disintegrin-like and metalloprotease with thrombospondin type I motif-5-mediated aggrecan degradation through syndecan-4 in intervertebral disc. The Journal of Biological Chemistry 286 (46): 39738–39749.
Wang, Z., D. Telci, and M. Griffin. 2011b. Importance of syndecan-4 and syndecan −2 in osteoblast cell adhesion and survival mediated by a tissue transglutaminase-fibronectin complex. Experimental Cell Research 317 (3): 367–381.
Wang, X., H. Wang, H. Yang, J. Li, Q. Cai, I.M. Shapiro, and M.V. Risbud. 2014. Tumor necrosis factor-alpha- and interleukin-1beta-dependent matrix metalloproteinase-3 expression in nucleus pulposus cells requires cooperative signaling via syndecan 4 and mitogen-activated protein kinase-NF-kappaB axis: Implications in inflammatory disc disease. The American Journal of Pathology 184 (9): 2560–2572.
Woods, A., R.L. Longley, S. Tumova, and J.R. Couchman. 2000. Syndecan-4 binding to the high affinity heparin-binding domain of fibronectin drives focal adhesion formation in fibroblasts. Archives of Biochemistry and Biophysics 374 (1): 66–72.
Wu, X., W. Liu, Z. Duan, Y. Gao, S. Li, K. Wang, Y. Song, Z. Shao, S. Yang, and C. Yang. 2016. The involvement of protease Nexin-1 (PN1) in the pathogenesis of intervertebral disc (IVD) degeneration. Scientific Reports 6: 30563.
Wuertz, K., and L. Haglund. 2013. Inflammatory mediators in intervertebral disk degeneration and discogenic pain. Global Spine Journal 3 (3): 175–184.
Zhang, Y., M. Pasparakis, G. Kollias, and M. Simons. 1999. Myocyte-dependent regulation of endothelial cell syndecan-4 expression. Role of TNF-alpha. The Journal of Biological Chemistry 274 (21): 14786–14790.
Zhang, X., Z. Hu, J. Hao, and J. Shen. 2016. Low intensity pulsed ultrasound promotes the extracellular matrix synthesis of degenerative human nucleus pulposus cells through FAK/PI3K/Akt pathway. Spine (Phila Pa 1976) 41 (5): E248–E254.
Funding
This study was supported by grants from the financial support of the National Science Foundation of China (NSFC, U1603121, 81201393, 81272025, 81541056; 2013YGYL015, WJ2017Z017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was reviewed and approved by the Ethics Committee of Tongji Medical College; written informed consent was obtained from all patients.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, X., Li, S., Wang, K. et al. TNF-α Regulates ITGβ1 and SYND4 Expression in Nucleus Pulposus Cells: Activation of FAK/PI3K Signaling. Inflammation 42, 1575–1584 (2019). https://doi.org/10.1007/s10753-019-01019-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-019-01019-9