Abstract—
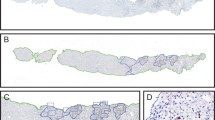
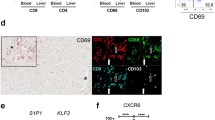
It remains unclear as to whether there are differences that exist in the types and functional status of immune cells within different areas of the liver lobules after rejection of liver transplantation. The composition of infiltrating T cells in liver allografts during liver transplantation rejection is indistinct and difficult to visualize within the same biopsy slide. In an attempt to rectify this problem, we applied multiplex immunofluorescent assays to assess the spatial distribution of various types of infiltrating T cells in different areas of the liver lobules after liver transplantation. In identical areas of the hepatic lobules, the percentage of CD4+ T, CD8+ T, and regulatory T (Treg) cells in the rejection group was greater than that observed in the non-rejection and normal groups. Within all three groups, the percentage of CD4+ T, CD8+ T, and Treg cells from the periportal to perivenous zones initially increased and then decreased. In the rejection group, the percentage of CD8+ T cells gradually increased from the periportal to perivenous zones, with maximal levels in the perivenous as compared with that in the transitional and periportal zones. In conclusion, levels of CD8+ T cells within different regions of liver lobules are closely related to levels of rejection after liver transplantation. Liver transplantation rejection may be linked with increases in CD8+ T cells within the perivenous zone. Although the regional percent of increase in CD4+ T cells may not reflect level of the rejection, the overall numbers of both of CD4+ and CD8+ T cells within different regions were closely related to rejection levels.




Similar content being viewed by others
References
Taner, T. 2017. Liver transplantation: Rejection and tolerance. Liver Transplantation 23 (S1): S85–S88.
Wei, Q., K. Wang, Z. He, et al. 2018. Acute liver allograft rejection after living donor liver transplantation: Risk factors and patient survival. American Journal of the Medical Sciences 356 (1): 23–29.
Duizendstra, A.A., M. Doukas, M. Betjes, et al. 2021. HLA matching and rabbit antithymocyte globulin as induction therapy to avoid multiple forms of rejection after a third liver transplantation. Clinics and Research in Hepatology and Gastroenterology 45 (3): 101539.
Chen, X., J. Zheng, J. Cai, et al. 2017. The cytoskeleton protein beta-actin may mediate T cell apoptosis during acute rejection reaction after liver transplantation in a rat model. American Journal of Translational Research 9 (11): 4888–4901.
Xie, X.J., Y.F. Ye, L. Zhou, et al. 2010. Th17 promotes acute rejection following liver transplantation in rats. Journal of Zhejiang University. Science. B 11 (11): 819–827.
Le Moine, A. 2021. What are the effects of everolimus and basiliximab on Tregs regulation and tolerance after liver transplantation? Clinics and Research in Hepatology and Gastroenterology 45 (5): 101591.
Haarer, J., P. Riquelme, P. Hoffmann, et al. 2016. Early enrichment and restitution of the peripheral blood tregs pool is associated with rejection-free stable immunosuppression after liver transplantation. Transplantation 100 (7): e39-40.
Steiner, P.E., and H.L. Ratcliffe. 1968. The hepatic lobules of suidae, tayassuidae, and hippopotamidae. Anatomical Record 160 (3): 531–538.
Chikamori, K., T. Araki, and M. Yamada. 1985. Pattern analysis of the heterogeneous distribution of succinate dehydrogenase in single rat hepatic lobules. Cellular and Molecular Biology 31 (3): 217–222.
Halpern, K.B., R. Shenhav, O. Matcovitch-Natan, et al. 2017. Single-cell spatial reconstruction reveals global division of labour in the mammalian liver. Nature 542 (7641): 352–356.
Sleyster, E.C., and D.L. Knook. 1982. Relation between localization and function of rat liver Kupffer cells. Laboratory Investigation 47 (5): 484–490.
Gola, A., M.G. Dorrington, E. Speranza, et al. 2021. Commensal-driven immune zonation of the liver promotes host defence. Nature 589 (7840): 131–136.
Okamura, A., T. Matsushita, A. Komuro, et al. 2020. Adipose-derived stromal/stem cells successfully attenuate the fibrosis of scleroderma mouse models. International Journal of Rheumatic Diseases 23 (2): 216–225.
Chen, Z., J. Liu, and D. Xu. 2021. Oncolytic adenovirus expressing CCL19 enhances immunity against gastric cancer in mice. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 37 (2): 119–124.
Mauldin, I.S., A. Mahmutovic, S.J. Young, and C.J. Slingluff. 2021. Multiplex immunofluorescence histology for immune cell infiltrates in melanoma-associated tertiary lymphoid structures. Methods in Molecular Biology 2265: 573–587.
Demetris, A.J., C. Bellamy, S.G. Hubscher, et al. 2016. 2016 Comprehensive update of the banff working group on liver allograft pathology: Introduction of antibody-mediated rejection. American Journal of Transplantation 16 (10): 2816–2835.
Rasmussen, S.N., H.H. Holm, J.K. Kristensen, and H. Barlebo. 1972. Ultrasonically-guided liver biopsy. British Medical Journal 2 (5812): 500–502.
Canadas, I., R. Thummalapalli, J.W. Kim, et al. 2018. Tumor innate immunity primed by specific interferon-stimulated endogenous retroviruses. Nature Medicine 24 (8): 1143–1150.
Crosbie, O.M., S. Norris, J.E. Hegarty, and C. O’Farrelly. 1998. T lymphocyte subsets and activation status in patients following liver transplantation. Immunological Investigations 27 (4–5): 237–241.
Natsuda, K., S. Eguchi, M. Takatsuki, et al. 2016. CD4 T lymphocyte counts in patients undergoing splenectomy during living donor liver transplantation. Transplant Immunology 34: 50–53.
Jiang, Z., Y. Chen, X. Feng, et al. 2013. Recipient cytotoxic T lymphocyte antigen 4 +49 single-nucleotide polymorphism is not associated with acute rejection after liver transplantation in Chinese population. International Journal of Medical Sciences 10 (3): 250–254.
Dong, J.Y., H. Yin, R.D. Li, et al. 2011. The relationship between adenosine triphosphate within CD4(+) T cells and acute rejection after liver transplantation. Clinical Transplantation 25 (3): E292–E296.
Wang, Y., M. Zhang, Z.W. Liu, et al. 2014. The ratio of circulating regulatory T cells (Tregss)/Th17 cells is associated with acute allograft rejection in liver transplantation. Plos One 9 (11): e112135.
Kamada, N., H.S. Davies, D. Wight, L. Culank, and B. Roser. 1983. Liver transplantation in the rat. Biochemical and histological evidence of complete tolerance induction in non-rejector strains. Transplantation 35 (4): 304–311.
Qian, S., L. Lu, Y. Li, et al. 1997. Apoptosis of graft-infiltrating cytotoxic T cells: A mechanism underlying “split tolerance” in mouse liver transplantation. Transplantation Proceedings 29 (1–2): 1168–1169.
Krukemeyer, M.G., J. Moeller, L. Morawietz, et al. 2004. Description of B lymphocytes and plasma cells, complement, and chemokines/receptors in acute liver allograft rejection. Transplantation 78 (1): 65–70.
Lee, C.W., Y.J. Ren, M. Marella, et al. 2020. Multiplex immunofluorescence staining and image analysis assay for diffuse large B cell lymphoma. Journal of immunological methods 478: 112714.
Francisco-Cruz, A., E.R. Parra, M.T. Tetzlaff, and I.I. Wistuba. 2020. Multiplex immunofluorescence assays. Methods in Molecular Biology 2055: 467–495.
Viratham, P.A., S.G. Craig, V. Bingham, et al. 2020. A robust multiplex immunofluorescence and digital pathology workflow for the characterisation of the tumour immune microenvironment. Molecular Oncology 14 (10): 2384–2402.
Parra, E.R., N. Uraoka, M. Jiang, et al. 2017. Validation of multiplex immunofluorescence panels using multispectral microscopy for immune-profiling of formalin-fixed and paraffin-embedded human tumor tissues. Science and Reports 7 (1): 13380.
Parra, E.R., J. Zhai, A. Tamegnon, et al. 2021. Identification of distinct immune landscapes using an automated nine-color multiplex immunofluorescence staining panel and image analysis in paraffin tumor tissues. Science and Reports 11 (1): 4530.
Calvani, J., M. Terada, C. Lesaffre, et al. 2020. In situ multiplex immunofluorescence analysis of the inflammatory burden in kidney allograft rejection: A new tool to characterize the alloimmune response. American Journal of Transplantation 20 (4): 942–953.
Porter, K.A. 1969. Pathology of liver transplantation. Transplantation Reviews 2: 129–170.
Lefkowitch, J.H. 2002. Diagnostic issues in liver transplantation pathology. Clinics in liver disease 6 (2): 555–570.
Stewart, B.J., J.R. Ferdinand, M.D. Young, et al. 2019. Spatiotemporal immune zonation of the human kidney. Science 365 (6460): 1461–1466.
Halpern, K.B., R. Shenhav, H. Massalha, et al. 2018. Paired-cell sequencing enables spatial gene expression mapping of liver endothelial cells. Nature Biotechnology 36 (10): 962–970.
Sablik, K.A., E.S. Jordanova, N. Pocorni, G.M. Clahsen-van, and M. Betjes. 2019. Immune cell infiltrate in chronic-active antibody-mediated rejection. Frontiers in Immunology 10: 3106.
Acknowledgements
The authors would like to thank the Key Laboratory of Transplantation immunity of Beijing for their technical support.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81970562).
Author information
Authors and Affiliations
Contributions
Shi-peng Li and Zhi-jun Zhu contributed to the research design. Guang-peng Zhou, Jie Sun, and Bin Cui collected the clinical data; Shi-peng Li and Jie Sun performed multiplex immunofluorescent assays and immunocytochemistry. Hai-ming Zhang, Lin Wei, and Li-ying Sun contributed to the data management and statistical analyses. Shi-peng Li and Zhi-jun Zhu wrote the manuscript. Shi-peng Li and Guang-peng Zhou contributed equally to this work.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
The study protocol was approved by the ethics committee of Beijing Friendship Hospital, Capital Medical University, Beijing, China.
Consent for Publication
All authors have reviewed the manuscript and have given consent for publication.
Availability of Data and Materials
All data generated or analyzed during this study are available in this article.
Competing Interests
The authors declare no conflicts of interest in this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Sp., Zhou, Gp., Sun, J. et al. Multiplex Immunofluorescence for Detection of Spatial Distributions of Infiltrating T Cells Within Different Regions of Hepatic Lobules During Liver Transplantation Rejection. Inflammation 45, 651–664 (2022). https://doi.org/10.1007/s10753-021-01574-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-021-01574-0