Abstract
Purpose
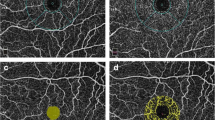
This study evaluated the macular ganglion cell–inner plexiform layer (GC-IPL) thickness using spectral-domain (SD) optical coherence tomography (OCT) in patients with chronic exposure to hydroxychloroquine (HCQ).
Methods
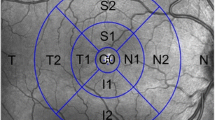
A total of 90 patients (90 eyes) treated with HCQ for at least 5 years and normal controls were included in the study. A fundus examination, automated threshold perimetry, and GC-IPL thickness measurements using the Cirrus high-definition OCT ganglion cell analysis algorithm were performed in all patients treated with HCQ. Average, minimum, and sectorial macular GC-IPL thicknesses were compared between the patients and controls.
Results
There was no statistically significant difference in age or sex between the groups. The anterior segment and fundoscopy were normal in all patients and controls. Visual field (VF) testing was normal in all patients. The average, minimum, and sectorial macular GC-IPL thicknesses were significantly lower in patients than those in control subjects.
Conclusions
There was significant thinning of the macular GC-IPL in the absence of clinically evident HCQ-related retinopathy and VF abnormalities. Measurements of the macular GC-IPL thickness using SD-OCT may therefore be useful in the early diagnosis and in monitoring the progression of retinal changes in patients receiving long-term HCQ therapy.
Similar content being viewed by others
References
Mavrikakis I, Sfikakis PP, Mavrikakis E, Rougas K, Nikolaou A, Kostopoulos C et al (2003) The incidence of irreversible retinal toxicity in patients treated with hydroxychloroquine: a reappraisal. Ophthalmology 110:1321–1326
Bienfang D, Coblyn JS, Liang MH, Corzillius M (2000) Hydroxychloroquine retinopathy despite regular ophthalmologic evaluation: a consecutive series. J Rheumatol 27:2703–2706
Levy GD, Munz SJ, Paschal J, Cohen HB, Pince KJ, Peterson T (1997) Incidence of hydroxychloroquine retinopathy in 1207 patients in a large multicenter outpatient practice. Arthritis Rheum 40:1482–1486
Tzekov R (2005) Ocular toxicity due to chloroquine and hydroxychloroquine: electrophysiological and visual function correlates. Doc Ophthalmol 110:111–120
Marmor MF, Carr RE, Easterbrook M, Farjo AA, Mieler WF (2002) Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: a report by the American Academy of Ophthalmology. Ophthalmology 109:1377–1382
Easterbrook M (1999) Detection and prevention of maculopathy associated with antimalarial agents. Int Ophthalmol Clin 39:49–57
Carr RE, Gouras P, Gunkel RD (1966) Chloroquine retinopathy: early detection by retinal threshold test. Arch Ophthalmol 75:171–178
Rosenthal AR, Kolb H, Bergsma D, Huxsoll D, Hopkins JL (1978) Choroquine retinopathy in the rhesus monkey. Invest Ophthalmol Vis Sci 17:1158–1175
Hallberg A, Naeser P, Andersson A (1990) Effects of long-term chloroquine exposure on the phospholipids metabolism in retina and pigment epithelium of the mouse. Acta Ophthalmol (Copenh) 68:125–130
Easterbrook M (1992) Long-term course of antimalarial maculopathy after cessation of treatment. Can J Ophthalmol 27:237–239
Michaelides M, Stover NB, Francis PJ, Weleber RG (2011) Retinal toxicity associated with hydroxychloroquine and chloroquine: risk factors, screening, and progression despite cessation of therapy. Arch Ophthalmol 129:30–39
Mititelu M, Wong BJ, Brenner M, Bryar PJ, Jampol LM, Fawzi AA (2013) Progression of hydroxychloroquine toxic effects after drug therapy cessation: new evidence from multimodal imaging. JAMA Ophthalmol 131:1187–1197
Marmor MF, Kellner U, Lai TY, Lyons JS, Mieler WF (2011) Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology 118:415–422
Gonasum LM, Potts AM (1974) In vitro inhibition of protein synthesis in the retinal pigment epithelium by chloroquine. Invest Ophthalmol Vis Sci 13:107–115
Geamănu Pancă A, Popa-Cherecheanu A, Marinescu B, Geamănu CD, Voinea LM (2014) Retinal toxicity associated with chronic exposure to hydroxychloroquine and its ocular screening. J Med Life 7:322–326
Palma Sánchez D, Rubio Velazquez E, Soro Marín S, Reyes García R (2013) Retinal toxicity due to antimalarials: frequency and risk factors. Reumatol Clin 9:259–262
Wolfe F, Marmor MF (2010) Rates and predictors of hydroxychloroquine retinal toxicity in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res (Hoboken) 62:775–784
Kim NR, Lee ES, Seong GJ, Kim JH, An HG, Kim CY (2010) Structure function relationship and diagnostic value of macular ganglion cell complex measurement using Fourier-domain OCT in glaucoma. Invest Ophthalmol Vis Sci 51:4646–4651
Cho JW, Sung KR, Lee S, Yun SC, Kang SY, Choi J et al (2010) Relationship between visual field sensitivity and macular ganglion cell complex thickness as measured by spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci 51:6401–6407
Mwanza JC, Oakley JD, Budenz DL, Chang RT, Knight OJ, Feuer WJ (2011) Macular ganglion cell–inner plexiform layer: automated detection and thickness reproducibility with spectral domain–optical coherence tomography in glaucoma. Invest Ophthalmol Vis Sci 52:8323–8329
Girkin CA, McGwin G Jr, Sinai MJ, Sekhar GC, Fingeret M et al (2011) Variation in optic nerve and macular structure with age and race with spectraldomain optical coherence tomography. Ophthalmology 118:2403–2408
Alasil T, Wang K, Keane PA, Lee H, Baniasadi N, de Boer JF et al (2013) Analysis of normal retinal nerve fiber layer thickness by age, sex, and race using spectral domain optical coherence tomography. J Glaucoma 22:532–541
Koh VT, Tham YC, Cheung CY, Wong WL, Baskaran M, Saw SM, Wong TY, Aung T (2012) Determinants of ganglion cell–inner plexiform layer thickness measured by high-definition optical coherence tomography. Invest Ophthalmol Vis Sci 53:5853–5859
Bonanomi MT, Dantas NC, Medeiros FA (2006) Retinal nerve fibre layer thickness measurements in patients using chloroquine. Clin Experiment Ophthalmol 34:130–136
Pasadhika S, Fishman GA (2010) Effects of chronic exposure to hydroxychloroquine or chloroquine on inner retinal structures. Eye (Lond) 24:340–346
Ulviye Y, Betul T, Nur TH, Selda C (2013) Spectral domain optical coherence tomography for early detection of retinal alterations in patients using hydroxychloroquine. Indian J Ophthalmol 61(4):168–171
Lee MG, Kim SJ, Ham DI, Kang SW, Kee C, Lee J et al (2014) Macular retinal ganglion cell–inner plexiform layer thickness in patients on hydroxychloroquine therapy. Invest Ophthalmol Vis Sci 25(56):396–402
Pasadhika S, Fishman GA, Choi D, Shahidi M (2010) Selective thinning of the perifoveal inner retina as an early sign of hydroxychloroquine retinal toxicity. Eye (Lond) 245:756–762
Marmor MF, Melles RB (2014) Disparity between visual fields and optical coherence tomography in hydroxychloroquine retinopathy. Ophthalmology 121:1257–1262
Uslu H, Gurler B, Yildirim A, Tatar MG, Aylin Kantarcı F, Goker H, Pehlevan HS, Colak HN (2016) Effect of hydroxychloroquine on the retinal layers: a quantitative evaluation with spectral-domain optical coherence tomography. J Ophthalmol 2016:8643174. doi:10.1155/2016/8643174
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Kan, E., Yakar, K., Demirag, M.D. et al. Macular ganglion cell–inner plexiform layer thickness for detection of early retinal toxicity of hydroxychloroquine. Int Ophthalmol 38, 1635–1640 (2018). https://doi.org/10.1007/s10792-017-0635-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-017-0635-y