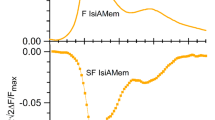
Steady-state and pulsed spectroscopic methods are used to study the spectroscopic and photophysical properties of the biologically important plant pigment rutin at room temperature and 77 K in organic solvents and a buffer solution at pH 7.0. The large dipole moment μe = 13.3 D of the rutin molecule in a Franck–Condon excited state indicates that rutin is dipolar in this excited state. The nonstationary S1 → Sn induced absorption spectra are characterized by a short-wavelength band at λabs max = 460 nm and low-intensity absorption in the 500–750 range which clearly belongs to associates of rutin. No residual induced absorption which might be related to triplet-triplet T1→Tk transitions in rutin was observed over the entire spectral range for times >50 ns. S1 → S0 fluorescence with a quantum yield Φfl ~ 10–4 was also observed at room temperature. The fluorescence and fluorescence excitation spectra manifest a weak dependence on the excitation and detection wavelengths, which may be related to the presence of conformers in the solution owing to rotation of the phenol B ring around a single 1′–2 bond. Lowering the temperature of a glassy frozen solution of rutin in ethanol to 77 K raises Φfl by a factor of 750. A rate constant kic = 3.7·1011 s–1 for internal conversion from the S1 state at room temperature is calculated from the spectral-luminescence data. It is found that the main channel for exchange of electronic excitation energy in the rutin molecule at room temperature is S1(π,π*) ~~> S0-internal conversion induced by the charge-transfer state.
Similar content being viewed by others
References
St. Rusnyak and A. Sent-Gyorgui, Nature, 138, 27 (1936).
J. A. Rothwell, A. J. Day, and M. R. A. Morgan, J. Agric. Food Chem., 53, 4355–4360 (2005).
A. Gaberščik, M. Vončina, T. Trošt, M. Germ, and L. O. Björn, J. Photochem. Photobiol. B: Biol., 66(b), 30–36 (2002).
K. E. Heim, A. R. Tagliaferro, and D. J. Bobilya, J. Nutrit. Biochem., 13, 572–584 (2002).
N. Saevan and A. Jimtaisong, J. Appl. Pharm. Science, 3, 129–141 (2013).
Z. G. Cerovic, G. Samson, F. Morales, N. Tremblay, and I. Moya, Agronomie, 19, 543–578 (1999).
E. Falkovskaia, P. K. Sengupta, and M. Kasha, Chem. Phys. Lett., 297, 109–114 (1998).
O. S. Wolfbeis, M. Leiner, P. Hochmuth, and H. Geiger, Ber. Bunsen-Ges. Phys. Chem., 88, 759–767 (1984).
P.-T. Chou, Y.-C. Chen, W.-S. Yu, and Y.-M. Cheng, Chem. Phys. Lett., 340, 89–97 (2001).
H.-B. Liu, D. Yu, S. C. Shin, H.-R. Park, J. K. Park, and K.-M. Bark, Photochem. Photobiol., 85, 934–942 (2009).
V. Ulvarosi, S. F. Barbuceanu, V. Aldea, C.-C. Arama, M. Baden, R. Olar, and D. Marinescu, Molecules, 15, 1578–1589 (2010).
S. L. Bondarev and V. N. Knyukshto, J. Lumin., 142, 236–240 ( 2013).
J. C. Valle, J. Chem. Phys. 124, 104506-1–104506-13 (2006).
M. Sarkar and P. K. Sengupta, J. Photochem. Photobiol. A: Chem., 48, 175–183 (1989).
E. C. Lim, J. Phys. Chem., 90, 6770–6777 (1986).
T. Lay, B. T. Lim, and E. C. Lim, J. Am. Chem. Soc., 104, 7631–7635 (1982).
G. J. Smith and K. R. Markham, J. Photochem. Photobiol. A: Chem., 99, 97–101 (1996).
E. A. Borisevich, V. N. Knyukshto, A. N. Kozyriev, and K. N. Solov′ev, Opt. Spektrosk., 74, 210–221 (1993).
J. N. Demas and G. A. Crosby, J. Chem. Phys., 75, 991–1012 (1971).
N. A. Borisevich, O. V. Buganov, S. A. Tikhomirov, G. V. Tolstorozhev, and G. L. Shkred, Kvant. Elektron., 28, 225–232 (1999).
T. J. Mabry, K. R. Markham, and M. B. Thomas, The Systematic Identifi cation of Flavonoids, Springer, Berlin (1970).
P. Matteini, A. Gotti, and G. Agati, Monatsch. Chem., 141, 793–800 (2010).
M. Friedman and H. S. Jürgens, J. Agric. Food Chem., 48, 2101–2110 (2000).
E. MacRae, J. Phys. Chem., 61, 562–570 (1957).
N. G. Bakhshiev, M. I. Knyazhanskii, V. I. Minkin, O. A. Osipov, and G. V. Saidov, Usp. Khim., 38, 1643–1670 (1969).
M. Ito, K. Inuzuka, and S. Imanisi, J. Am. Chem. Soc., 82, 1317–1322 (1960).
V. Sadovoy, A. Silantyev, M. Selimov, and T. Shchedrina, Food Nutr. Sci., 2, 1121–1127 (2011).
A. Bondi, J. Phys. Chem., 68, 441–451 (1964).
H. Reinhardt, Solvents in Organic Chemistry [in Russian], Khimiya, Leningrad (1973).
R. H. M. van de Leur, Polymer, 35, 2691–2700 (1994).
Y. Norikane, H. Itoh, and T. Arai, J. Photochem. Photobiol. A: Chem., 161, 163–168 (2004).
V. Avila and C. M. Previtali, J. Chem. Soc. Perkin Trans., 2 (12), 2281–2286 (1995).
G. R. Fleming, in: Chemical Applications of Ultrafast Spectroscopy, Oxford Univ. Press, New York (1986), pp. 179–195.
I. Yu. Martynov, A. B. Demyashkevich, B. M. Uzhinov, and M. G. Kuz′min, Usp. Khim., 46, 3–28 (1977).
V. G. Plotnikov and G. V. Maier, Opt. Spektrosk., 47, 113–120 (1979).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Prikladnoi Spektroskopii, Vol. 82, No. 6, pp. 852–859, November–December, 2015.
Rights and permissions
About this article
Cite this article
Bondarev, S.L., Knyukshto, V.N., Tikhomirov, S.A. et al. Mechanism for Highly Efficient Non-Radiative Deactivation of Electronic Excitation in Rutin. J Appl Spectrosc 82, 929–935 (2016). https://doi.org/10.1007/s10812-016-0207-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-016-0207-3