Abstract
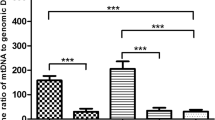
Purpose: To perform preimplantation genetic diagnosis (PGD) of Leigh encephalopathy, we developed a rapid and reliable quantification assay for the percentage of T8993G mtDNA mutation and analyzed various specimens. Methods: We prepared the standard curve by measuring serial proportion of 8993T/G cloned plasmid DNA using real-time PCR, and measured (1) mutant DNA (known proportions by PCR-RFLP), (2) single lymphocytes from 46% mutant carrier, (3) 123 blastomeres from 20 abnormal embryos. Results: (1) These were within −5∼+6% error range, (2) mean 44.3%(11–70%), (3) Five embryos harbored T8993G mutation (4–22%). Embryos from same person indicated different degrees of heteroplasmy, and blastomeres from same embryo demonstrated limited dispersion of heteroplasmy (2–11%). Conclusions: (1) This method provides rapid and reliable PGD for Leigh encephalopathy. (2) The variable heteroplasmy with somatic mitosis was suggested. (3) T8993G mutation was existed in undeveloped embryo, and the bottleneck theory was supported. The limited heteroplasmy dispersion of blastomeres from same embryo also supported reliability of PGD for T8993G mutation.






Similar content being viewed by others
References
Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJ, Staden R, Young IG. Sequence and organization of the human mitochondrial genome. Nature 1981;290:457–65.
Cummins J. Mitochondrial DNA in mammalian reproduction. J Reprod Fertil 1998;3:172–82.
DiMauro S. Mitochondrial encephalomyopathies. In: Rosenberg RN, Prusiner SB, DiMauro S, eds. The molecular and genetic basis of neurological disease. Boston: Butterworth-Heinemann; 1993, pp. 665–94.
Shoffner JM, Wallace DC. Oxidative phosphorylation diseases. In: Scriver CR, Beaudet AL, Sly WS, Valle MD, eds. The metabolic and molecular bases of inherited disease, 7th ed, New York: McGraw Hill; 1995, pp. 1535–609.
Hauswirth WW, Laipis PJ. Mitochondrial DNA polymorphism in a maternal lineage of Holstein cows. Proc Natl Acad Sci USA 1982;79:4686–90.
Hauswirth WW, Laipis PJ. Transmission genetics of mammalian mitochondria: a molecular model and experimental evidence. In: Quagliariello E, Slater EC, Palmieri F, eds. Achievements and perspectives of Mitochodrial Research. Amsterdam: Elsevier, 1985, Vol. II, pp. 49–59.
Laipis PJ, Van de Walle MJ, Hauswirth WW. Unequal partitioning of bovine mitochondrial genotypes among siblings. Proc Natl Acad Sci USA 1988;85:8107–10.
Ashley MV, Laipid PJ, Hauswirth WW. Rapid segregation of heteroplasmic bovine mitochondria. Nucleic Acids Res 1989;17:7325–31.
Koehler CM, Lindberg GL, Brown DR, Beitz DC, Freeman AE, Mayfield JE, Myers AM. Replacement of bovine mitochondrial DNA by a sequence variant within one generation. Genetics 1991;129:247–55.
Chinnery PF, Howell N, Lightowlers RN, Turnbull DM. Molecular pathology of MELAS and MERRF: the relationship between mutation load and clinical phenotype. Brain 1997;120:1713–21.
Chinnery PF, Howell N, Lightowlers RN, Turnbull DM. MELAS and MERRF: the relationship between maternal mutation load and the frequency of clinically affected offspring. Brain 1998;121:1889–94.
White SL, Shanske S, McGill JJ, Mountain H, Geraghty MT, DiMauro S, Dahl HH, Thorburn DR. Mitochondrial DNA mutations at nucleotide 8993 show a lack of tissue- or age-related variation. J Inherit Metab Dis 1999;22:899–914.
Dahl HH, Thorburn DR, White SL. Towards reliable prenatal diagnosis of mtDNA point mutations: studies of nt8993 mutations in oocytes, fetal tissues, children and adults. Hum Reprod 2000;15:246–55.
Holt IJ, Harding AE, Petty RK, Morgan-Hughes JA. A new mitochondrial disease associated with mitochondrial DNA heteroplasmy. Am J Hum Genet 1990;46:428–33.
Santorelli FM, Shanske S, Macaya A, DeVivo DC, DiMauro S. The mutation at nt 8993 of mitochondrial DNA is a common cause of Leith’s syndrome. Ann Neurol 1993;34:827–34.
Rahman S, Blok RB, Dahl HH, Danks DM, Kirby DM, Chow CW, Christodoulou J, Thorburn DR. Leigh syndrome: clinical features and biochemical and DNA abnormalities. Ann Neurol 1996;39:343–51.
Makela-Bengs P, Suomalainen A, Majander A, Rapola J, Kalimo H, Nuutila A, Pihko H. Correlation between the clinical symptoms and the proportion of mitochondrial DNA carrying the 8993 point mutation in the NARP syndrome. Pediatr Res 1995;37:634–9.
White SL, Collins VR, Wolfe R, Cleary MA, Shaske S, DiMauro S, Dahl HH, Thorburn DR. Genetic counseling and prenatal diagnosis for the mitochondrial DNA mutations at nucleotide 8993. Am J Hum Genet 1999;65:474–82.
Steffann J, Frydman N, Gigarel N, Burlet P, Ray PF, Fanchin R, Feyereisen E, Kerbrat V, Tachdjian G, Bonnefont JP, Frydman R, Munnich A. Analysis of mtDNA variant segregation during early human embryonic development: a tool for successful NARP preimplantation diagnosis. J Med Genet 2006;43:244–7.
Makino M, Horai S, Goto Y, Nonaka I. Mitochondrial DNA mutations in Leigh syndrome and their phylogenetic implications. J Hum Genet 2000;45:69–75.
Bourgeron T, Chretien D, Rotig A, Munnich A, Rustin P. Prenatal diagnosis of cytochrome c oxidase deficiency in cultured amniocytes is hazardous. Prenat Diagn 1992;12:548–9.
Dean NL, Battersby BJ, Ao A, Gosden RG, LinTan SL, Shoubridge EA, Molnar MJ. Prospect of preimplantation genetic diagnosis for heritable mitochondrial DNA diseases. Mol Hum Rep 2003;9:631–8.
Blok RB, Gook DA, Thorburn DR, Dahl HH. Skewed segregation of the mtDNA nt 8993(T→G) mutation in human oocytes. Am J Hum Genet 1997;60:1495–501.
Acknowledgments
I appreciate my coworkers’ collaborations and advice on the study, especially Ms. Yoko Yasuda, Ms. Maya Higuchi, Ms. Satoko Moriya, and Ms. Yuko Matumoto, and also alongside the financial and institutional support from the Department of Obstetrics and Gynecology, Keio University School of Medicine.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tajima, H., Sueoka, K., Moon, S.Y. et al. The development of novel quantification assay for mitochondrial DNA heteroplasmy aimed at preimplantation genetic diagnosis of Leigh encephalopathy. J Assist Reprod Genet 24, 227–232 (2007). https://doi.org/10.1007/s10815-007-9114-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-007-9114-0