Abstract
Purpose
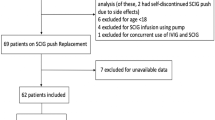
Utilization reports on immunoglobulin (Ig) use for immunodeficiency in the United States (U.S.) have focused on prescribing practices in hospitals. There have been no large-scale reports on Ig use for immune deficiency in the home. We investigated the use of Ig in 3,187 subjects diagnosed with primary immunodeficiency.
Methods
Cross-sectional data on 4,580 subjects in the U.S. receiving Ig in 2011 was obtained from a major home care provider. Demographics, route, dose, and frequency of Ig use by subjects with ICD-9 coded primary immunodeficiencies were analyzed.
Results
Of 4,580 subjects, 3,187 had ICD-9 codes suggesting primary immunodeficiencies; 1,939 (60.8 %) were females and 1,248 (39.2 %) were males, with age ranging from 0 to 95 years. The predominant diagnoses were: common variable immunodeficiency (279.06; n = 1,764; 55.3 %), hypogammaglobulinemia (279.00; n = 635; 19.9 %), unspecified immunity deficiency (279.3; n = 286; 9 %), other selective Ig deficiencies (279.03; n = 171; 5.4 %), and agammaglobulinemia (279.04; n = 127; 4 %). 54 % of subjects received Ig by the subcutaneous (SC) route, and 46 % by intravenous (IV) route, with more SC use by older subjects. The mean dose prescribed was 483 mg/kg/month, but less Ig was ordered for subjects on SCIg (409 mg/kg/month), as compared to subjects on IVIg (568 mg/kg/month). A highly significant inverse correlation between increasing age and dosage of Ig ordered was found (P = <.0001).
Conclusion
Analysis of home care use of Ig in primary immune deficiency revealed that the SC route was prescribed more than the IV route, especially for older patients. By either method of administration, less immunoglobulin was prescribed for older subjects.


Similar content being viewed by others
References
Darabi K, Abdel-Wahab O, Dzik WH. Current usage of intravenous immune globulin and the rationale behind it: the Massachusetts General Hospital data and a review of the literature. Transfusion. 2006;46(5):741–53.
Orange JS, Hossny EM, Weiler CR, Ballow M, Berger M, Bonilla FA, et al. Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2006;117(4 Suppl):S525–53.
Shehata N, Palda V, Bowen T, Haddad E, Issekutz TB, Mazer B, et al. The use of immunoglobulin therapy for patients with primary immune deficiency: an evidence-based practice guideline. Transfus Med Rev. 2010;24 Suppl 1:S28–50.
Lucas M, Lee M, Lortan J, Lopez-Granados E, Misbah S, Chapel H. Infection outcomes in patients with common variable immunodeficiency disorders: relationship to immunoglobulin therapy over 22 years. J Allergy Clin Immunol. 2010;125(6):1354–60.e4.
Berger M. Incidence of infection is inversely related to steady-state (trough) serum IgG level in studies of subcutaneous IgG in PIDD. J Clin Immunol. 2011;31(5):924–6.
Chapel HM, Spickett GP, Ericson D, Engl W, Eibl MM, Bjorkander J. The comparison of the efficacy and safety of intravenous versus subcutaneous immunoglobulin replacement therapy. J Clin Immunol. 2000;20(2):94–100.
Gardulf A, Nicolay U, Asensio O, Bernatowska E, Bock A, Carvalho BC, et al. Rapid subcutaneous IgG replacement therapy is effective and safe in children and adults with primary immunodeficiencies—a prospective, multi-national study. J Clin Immunol. 2006;26(2):177–85.
Maarschalk-Ellerbroek LJ, Hoepelman IM, Ellerbroek PM. Immunoglobulin treatment in primary antibody deficiency. Int J Antimicrob Agents. 2011;37(5):396–404.
Bonagura VR, Marchlewski R, Cox A, Rosenthal DW. Biologic IgG level in primary immunodeficiency disease: the IgG level that protects against recurrent infection. J Allergy Clin Immunol. 2008;122(1):210–2.
Gajewski LK, Bailey EM, Brown PD, Chandrasekar PH. Immune globulin use at a multihospital medical center. Am J Hosp Pharm. 1994;51(6):801–5.
Chen C, Danekas LH, Ratko TA, Vlasses PH, Matuszewski KA. A multicenter drug use surveillance of intravenous immunoglobulin utilization in US academic health centers. Ann Pharmacother. 2000;34(3):295–9.
Frauger E, Grassi J, Pradel V, Bornet C, Rouby F, Delorme J, et al. Use of intravenous immunoglobulins in clinical practice: data from three French university hospitals. Fundam Clin Pharmacol. 2011;25(6):753–61.
Gathmann B, Grimbacher B, Beaute J, Dudoit Y, Mahlaoui N, Fischer A, et al. The European internet-based patient and research database for primary immunodeficiencies: results 2006–2008. Clin Exp Immunol. 2009;157 Suppl 1:3–11.
Roifman CM, Levison H, Gelfand EW. High-dose versus low-dose intravenous immunoglobulin in hypogammaglobulinaemia and chronic lung disease. Lancet. 1987;1(8541):1075–7.
Liese JG, Wintergerst U, Tympner KD, Belohradsky BH. High- vs low-dose immunoglobulin therapy in the long-term treatment of X-linked agammaglobulinemia. Am J Dis Child. 1992;146(3):335–9.
Eijkhout HW, van Der Meer JW, Kallenberg CG, Weening RS, van Dissel JT, Sanders LA, et al. The effect of two different dosages of intravenous immunoglobulin on the incidence of recurrent infections in patients with primary hypogammaglobulinemia. A randomized, double-blind, multicenter crossover trial. Ann Intern Med. 2001;135(3):165–74.
Nelson Jr RP, Ballow M. 26. Immunomodulation and immunotherapy: drugs, cytokines, cytokine receptors, and antibodies. J Allergy Clin Immunol. 2003;111(2 Suppl):S720–43.
Yong PL, Boyle J, Ballow M, Boyle M, Berger M, Bleesing J, et al. Use of intravenous immunoglobulin and adjunctive therapies in the treatment of primary immunodeficiencies: a working group report of and study by the Primary Immunodeficiency Committee of the American Academy of Allergy Asthma and Immunology. Clin Immunol. 2010;135(2):255–63.
Ochs HD, Gupta S, Kiessling P, Nicolay U, Berger M. Safety and efficacy of self-administered subcutaneous immunoglobulin in patients with primary immunodeficiency diseases. J Clin Immunol. 2006;26(3):265–73.
Hagan JB, Fasano MB, Spector S, Wasserman RL, Melamed I, Rojavin MA, et al. Efficacy and safety of a new 20 % immunoglobulin preparation for subcutaneous administration, IgPro20, in patients with primary immunodeficiency. J Clin Immunol. 2010;30(5):734–45.
Wasserman RL, Irani AM, Tracy J, Tsoukas C, Stark D, Levy R, et al. Pharmacokinetics and safety of subcutaneous immune globulin (human), 10 % caprylate/chromatography purified in patients with primary immunodeficiency disease. Clin Exp Immunol. 2010;161(3):518–26.
Berger M, Rojavin M, Kiessling P, Zenker O. Pharmacokinetics of subcutaneous immunoglobulin and their use in dosing of replacement therapy in patients with primary immunodeficiencies. Clin Immunol. 2011;139(2):133–41.
Bonilla FA. IgG replacement therapy, no size fits all. Clin Immunol. 2011;139(2):107–9.
Acknowledgments
We gratefully acknowledge the help of Keith Crawford, Senior VP, Medical Marketing, Manufacturer Affairs, & Clinical Trials at Coram Specialty Infusion Services for giving us access to this data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, F., Feuille, E. & Cunningham-Rundles, C. Home Care Use of Intravenous and Subcutaneous Immunoglobulin for Primary Immunodeficiency in the United States. J Clin Immunol 33, 49–54 (2013). https://doi.org/10.1007/s10875-012-9776-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-012-9776-y