Abstract
Four new network organic–inorganic hybrid supramolecular compounds [PW12O40](C2H4N3)3·6H2O (1), [PMo12O40](C2H4N3)3·6H2O (2), [H4SiW12O40]8[C6NO2H4]4[C6NO2H5]16[C5NH6]4·39H2O (3) and [H3VW12O40] (C6H6NO2)2(CHO2)2·4H2O (4) composed by keggin type heteropolyanion and O/N-containing organic groups of 1H-1,2,4-Triazole or 2,3-Pyridinedicarboxylic acid have been successfully synthesized by hydrothermally method, and characterized by infrared spectrum (IR), thermogravimetric–differentialthermal analysis (TG–DTA), cyclic voltammetry (CV) and single crystal X-ray diffraction (XRD). Compounds 1–4 exhibit three dimensional supramolecular network via hydrogen bonds and/or π–π stacking interactions. These compounds exhibit good thermal stability and catalytic ability. They are active for catalytic oxidation of methanol in a continuous-flow fixed-bed micro-reactor, when the initial concentration of methanol is 2.5 g m−3 in air and flow rate is 10 mL min−1, the corresponding elimination rates of methanol are 65% (125 °C), 85% (125 °C), 94% (150 °C), and 80% (125 °C), respectively.
Similar content being viewed by others
Introduction
Stimulated by the fascinating applications in the fields of photochemistry [1], magnetism [2], efficient adsorbents [3], macromolecular crystallography [4], medicine [5], catalysis [6] as well as by the intriguing structural features, polyoxometalates (POMs) materials have attracted great interest in recent years. Several strategies have been considered to modify POMs while retaining their structural integrity, intrinsic properties, and facilitating their implementation into extended structures. The mostly extended POMs are generalized as POM-based inorganic–organic hybrid compounds, in which POMs are used as available and controllable secondary building units (SBUs) to construct intriguing hybrid compound [7]. The study on functionalization of POMs has became a significant direction to develop new inorganic–organic hybrid materials with useful catalytic, optical, electronic and magnetic properties.
Up to now, a number of POM-based inorganic–organic hybrid compounds have been prepared and characterized [8,9,10,11,12,13]. It is noted that POM-based inorganic–organic hybrids can be classified conveniently into two main classes, according to the main chemical interactions between the inorganic (POM) and organic/metal–organic components. Type I inorganic–organic hybrids refer to the situation where only non-covalent weak interactions [14,15,16,17] (e.g. π–π stacking, hydrogen bonding, electrostatic, van der waals force and so on) are existed between two components, which can generate structures defined as “supramolecular synthons” and is crucial to the topochemical reactivity of molecules in the crystalline state [18]; type II POMs and S/O/N-containing groups are linked with covalent bond, forming covalent interactions between inorganic (POM) and organic groups. While the type II can be subdivided into two models: (i) the linkages of POMs and S/O/N-containing groups are directly without mediums [13]; (ii) POMs and organic parts are combined via the help of medium metal atoms [17, 19, 20]. Although many POM-based inorganic–organic hybrids have been reported, it is still a challenging and meaningful work to obtain novel structure crystalline hybrid compounds and research their applications.
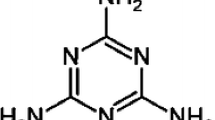
On account of these reasons, we also dedicate to the syntheses of novel organic–inorganic hybrid compounds based on polyoxometalates. According to the ability to accept electrons of heteropolyanions, we chose a kind of N-heterocyclic type molecules of 1H-1,2,4-Triazole and 2,3-Pyridinedicarboxylic acid to construct organic–inorganic hybrid compounds with keggin-type heteropolyanions. Here we report the hydrothermal synthesis, crystal structure and catalytic property of four new organic–inorganic polyoxometalates: [PW12O40](C2H4N3)3·6H2O (1), [PMo12O40] (C2H4N3)3·6H2O (2), [H4SiW12O40]8[C6NO2H4]4[C6NO2H5]16[C5NH6]4·39H2O (3) and [H3VW12O40](C6H6NO2)2(CHO2)2·4H2O (4). The elimination of methanol was used as the probe reaction to evaluate the catalytic activity of these compounds.
Experimental
Materials and Methods
All reagents were purchased commercially and used without further purification.
C, H, and N elemental analyses were performed on a Perkin-Elmer 240C elemental analyzer; other elemental analyses were performed on a Jarrell-Ash 1100 + 2000 ICP quantometer. The crystal structures of compounds 1–4 were determined with a Bruker Smart Apex II CCD area detector single-crystal diffractometer (Bruker, Germany) with graphite-monochromatized Mo Kα (λ = 0.71073 Å) radiation by the ψ–ω scan method. Thermogravimetric–differential thermal (TG–DTA) data of the samples were obtained on a WCT-1D instrument (Beijing optical instrument factory, Beijing, China) from room temperature to 800 or 1000 °C under air atmosphere, heating-rate was 10 °C min−1. Cyclic voltammetry (CV) curves were tested on Electrochemical work station CH1760C of Shanghai Chen-Hua Instrument with the scan rate of 20 mV s−1, two platinum electrode been used as the working electrode and the auxiliary electrode, respectively, the calomel electrode been used as the reference electrode.
Synthesis
Synthesis of Compound 1
A mixture of Na3PO4·12H2O (0.114 g, 0.3 mmol), NaVO3·2H2O (0.366 g, 0.3 mmol), Na2WO4·2H2O (0.4948 g, 1.5 mmol) was dissolved in 15 mL distilled water, and heat to dissolve. Then the pH of the solution was adjusted to 1.75 with 1:1 (V: V) H2SO4, the bright red solution was obtained after stirring for 30 min. The 1H-1,2,4-triazole (0.0207 g, 0.3 mmol) was added into above solution. Then the mixed solution was sealed in a 20 mL Teflon-lined autoclave at 170 °C for 96 h, then the autoclave was cooled to room temperature at 10 °C h−1. Golden yellow block crystals were filtered and washed with distilled water. Yield: 36.2% (based on W). Anal. Calcd for 1, C, 2.26; H, 0.76; N, 3.95; P, 0.97; W, 69.09%. Found: C, 2.15; H, 0.72; N, 3.81; P, 0.83; W, 68.60%.
Synthesis of Compound 2
The synthetic procedure was similar to that of 1 except that Na2MoO4·2H2O was used instead of Na2WO4·2H2O, and pH was adjusted to 2.00. Jacinth cubic crystals were obtained in about 57.2% yield (based on Mo). Anal. Calcd for 2, C, 3.37; H, 1.13; N, 5.89; P, 1.45; Mo, 53.78%. Found: C, 3.30; H, 1.02; N, 5.74; P, 1.39; W, 53.65%.
Synthesis of Compound 3
The synthetic procedure was similar to that of 1 except that Na3SiO4·12H2O and 2,3-pyridine dicarboxylic acid were used instead of Na3PO4·2H2O and 1H-1,2,4-triazole, respectively yellow block crystals were obtained. Yield: 45% (based on W). Anal. Calcd for 3, C, 6.34; H, 0.87; N, 1.27; Si, 0.85; W, 66.56%. Found: C, 6.25; H, 0.79; N, 1.18; Si, 0.77; W, 66.48%.
Synthesis of Compound 4
The synthetic procedure was similar to that of 1 except that 2,3-pyridine dicarboxylic acid was used instead of 1,2,4-triazole, respectively. Orange red block crystals were obtained. Yield: 50.5% (based on W). Anal. Calcd for 4, C, 5.1; H, 0.76; N, 0.85; V, 1.54; W, 66.6%. Found: C, 4.9; H, 0.68; N, 0.76; V, 1.47; W, 66.52%.
It’s worth noting that starting material elements (V for 1–3, P for 2) were not included to the proposed structures, but without them the products could not obtain, which may be act as a template agent or a trigger agent in the process of the reaction.
X-Ray Crystallography
The structures of compounds 1–4 were determined by a Bruker SMART Apex II CCD diffractometer equipped with a graphite-monochromated MoKα (λ = 0.71073 Å) radiation by the ψ–ω scan method at 298 K. All absorption corrections were performed with the SADABS program [21]. The structure was solved by direct methods and refined by full-matrix least-squares on F2 using the Shelxtl-97 [22] program package. All non-hydrogen atoms were finally refined with anisotropic displacement parameters. All hydrogen atoms of the crystal water were added by different Fourier methods while other hydrogen atoms were finally refined as a riding mode using the default Shelxtl parameters. The crystallographic details of 1–4 are summarized in Table 1. Crystallographic data for the structures reported in this paper have been deposited in the Cambridge Crystallographic Data Center with CCDC reference numbers 1512957, 1512958, 1512959 and 1512960 for compounds 1–4, respectively.
Catalytic Activity
The catalytic activity of compounds 1–4 were investigated for elimination of methanol gases.
Catalytic reactions were carried out in a continuous-flow fixed-bed micro-reactor (Fig. S1). The catalytic properties of compounds were tested by eliminating the methanol. 0.2 g compound was loaded in the catalytic reaction tube (φ/8 mm; L/200 mm) as catalyst. The simulacrum of polluted air containing reaction substrate of methanol was prepared by bubbling clean air into a container filled with reaction substrate and diluting the gas with clean dry air. The initial concentration of reaction substrate was dominated by adjusting the flow velocity of the bubbling gas and dilution air. The feed gas flow through the reactor, the catalytic elimination reaction carried on at different temperature, which controlled by a thermostat. The substrate concentration was detected by online gas chromatograph (GC-102 M, FID detector) once in every 5 min until the concentration unchanged which means the reaction reaches balance. The inorganic products of the effluent gases were monitored by a PdCl2 [0.2 (wt %)] solution and saturated lime-water solution.
Blank experiments were carried out without the catalysts.
Results and Discussion
Crystal Structure of Compounds
Structure of Compound 1 and 2
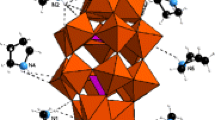
Compounds 1 and 2 are isostructural and both crystallize in the R-3 space group. Compound 1 [PW12O40](C2H4N3)3·6H2O consists of one typical keggin polyoxoanion [PW12O40]3−, three protonated cations [C2H4N3]+ and six water molecules. The asymmetric structure unit of Compound 1 is shown in Fig. 1a.
[PW12O40]3− is a regular keggin type polyoxoanion, which contains a center PO4 tetrahedron and four W3O13 three metal clusters. Three WO6 octagonal are linked by the edge to form a W3O13 group, while four W3O13 groups and one PO4 center tetrahedron are linked by angle. The P–Oa(central oxygen) distance in the range of 1.49(3)–1.509(14) Å, and the Oa–P–cOa angle is in the range of 108.9(6)–110.0(6)°, suggesting the formation of a micro-distortion tetrahedron. The W–Od (terminal oxygen), W-Oa, W-Ob/c(bridge oxygen) distances fall in the ranges of 1.691(14)–1.720(14) Å, 2.425(14)–2.463(15) Å and 1.864(14)–1.958(15) Å, respectively, and the O–W–O angle of the axial direction is in the range of 71.7(5)–172.6(6)°, indicating that WO6 are distorted octahedrons.
On the crystallography the polyoxoanion [PW12O40]3−, protonated cations [C2H3N3]+ and water molecules are independent. While on the molecular level they are a whole, which is mainly assembled by non-covalent bonds of hydrogen bonds and electrostatic interaction forming charge-transfer hybrid involving organic donors and inorganic acceptors. It is worth noting that via hydrogen-bonding, the numerous cations in 1 are linked together to form hole-net intersected channels with dimension of about 10.67 Å × 10.06 Å, in which the [PW12O40]3− anions resided (Fig. 1b).
The asymmetric structure unit drawing of compound 2 is shown in supplementary Fig. S2, the empirical formula of compound 2 is [PMo12O40](C2H4N3)3·6H2O, which consists of one typical keggin polyoxoanion [PMo12O40]3−, three protonated cations [C2H4N3]+ and six water molecules, just as 1.
Structure of Compound 3
Compound 3 [H4SiW12O40]8[C6NO2H4]4[C6NO2H5]16[C5NH6]4·39H2O is a large-scale complicated molecule, which is constructed by eight typical Keggin-type protonated [H4SiW12O40] units, four 3-pyridine carboxylic acid anion [C6O2NH4]−, sixteen neutral 3-pyridine carboxylic acid [C6NO2H5], four protonated pyridine [C5NH6]+ cations and thirty-nine water molecules. The asymmetric structure unit of compound 3 is shown in Fig. 2.
It’s worth noting that the centroid distance of the two aromatic rings fall in the ranges of 3.548–5.859 Å (Table S1), which indicates that some π–π stacking interaction are existed in the compound 3, but the distance between interacting planes is too far and the π–π stacking interaction is too weak.
On the crystallography these compositions are independent, which are main linked by supramolecular interactions of hydrogen bonds electrostatic interaction and π–π stacking interaction forming supramolecular compound (Fig. S3).
Structure of Compound 4
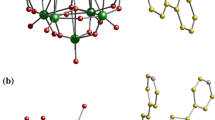
The crystal structure analysis reveals that compound 4 [H3VW12O40](C6H6NO2)2(CHO2)2·4H2O consists of one neutral [H3VW12O40] unit, two protonated 3-pyridine carboxylic acid groups [C6H6O2N]+, two formate ions [CHO2]− and four water molecules, divided into four sets of independent molecules in an unit cell. It’s worth nothing that [C6H6O2N]+ and [CHO2]− come from the decomposition of 2,3-pyridine dicarboxylic acid. The [VW12O40]3− is constituted by the central V atom and 12 WO6 octagonal. The central V atom is surrounded by a distorted cube constituted by eight half-occupied oxygen atoms (Fig. 3b) at 1.480(18)–1.59(2) Å (mean 1.535 Å). The structure feature of disorder often appears in the keggin structure [23,24,25]. The W–Od, W–Oa, W–Ob/c bonds are in the usual range of 1.636(8)–1.667(8) Å, 2.421(14)–2.481(17) Å and 1.844(8)–1.920(9) Å, respectively. All W–O distances are similar with that of typical normal keggin type of polyoxoanion. It’s worth noting that the POM group of idealized Td symmetry lies on a special position of point-group symmetry while incompatibility between these symmetries results in positional disorder just as compound 4.
There are a lot of intramolecular and intermolecular hydrogen bonds in compounds 1–4, which play an important role in consolidating the crystal architecture and forming 3D supermolecular compound. It’s worth noting that in the compound 3 and 4 organic groups are not 2,3-pyridine dicarboxylic acid, but the decomposition products of 2,3-pyridine dicarboxylic acid, which may be that in the high temperature and pressure hydrothermal conditions 2,3-pyridine dicarboxylic acid is unstable that easy undergo decarboxylation.
IR Spectrum
The IR spectra of 1–4 have been recorded between 4000 and 400 cm−1 with KBr pellets, which exhibit the characteristic vibration patterns derived from keggin framework in the low-wavenumber region 700–1100 cm−1 [26] (Fig. S4). Four characteristic vibration bands attributable to ν(M–Od), ν(N–Oa), ν(M–Ob), and ν(M–Oc) (M = W(1, 3, 4), Mo(2); N = P(1, 2), Si(3), V(4)) are observed at 1081, 989, 893, and 795 cm−1 for 1; 1063, 959, 858, and 785 cm−1 for 2; 1015, 980, 914 and 804 cm−1 for 3; 1069, 969, 891, and 798 cm−1 for 4, respectively.
Thermogravimetric Differential Thermal
The TG–DTA curve of compound 1 is shown in Fig. S5a. The TG curve of compound 1 exhibits three steps of weight losses. The first weight loss is 6.25% in the temperature range of 25–102 °C, accompanied with an obvious endothermic peak at around 98 °C, corresponding to the release of non-coordinated and absorbed water molecules. The second weight loss is 8.75% from 339 to 477 °C, with a sharp exothermic peak at around 410 °C, corresponding to the release of 1,2,4-triazole units. The third step weight loss is 2.72% in the temperature range of 487–594 °C, assigned to the decomposition of [PW12O40]3−. The total weight loss of compound 1 is 11.47% in the range of 25–800 °C (except the absorbed water weight loss 6.25%), which is very close to calculated ones (12.88%).
The TG–DTA curve of 2 is shown in Fig. S5b. It shows a huge total weight loss of 72.72% in the range of 25–1000 °C. The weight loss of 12.66% at 25–120 °C, with an endothermic peak at around 95 °C, corresponds to the loss of the non-coordinated and the absorbed water molecules. The weight loss of 13.41% at 220–500 °C, with two endothermic peaks at around 400 and 450 °C, corresponds to the decomposition and combustion of 1,2,4-triazole organic groups. A big weight loss of 47.17% appears at 750–1000 °C, accompanied with two concomitant endothermic peaks in the DTA curve (765, 786 °C), corresponding to the decomposition of [PMo12O40]3− and then sublimation of some P2O5 and MoO3.
The TG–DTA curve of 3 is shown in Fig. S5c. It shows a total weight loss of 38.43% in the range of 25–800 °C. The weight loss of about 20% at 25–240 °C corresponds to the loss of the absorbed water molecules, accompanied with an endothermic peak at around 100 °C. The weight loss of 10% at 240–436 °C, accompanied with an endothermic peak at around 375 °C, corresponds to crystal phase transition of compound and the release structural water molecules. The last one weight loss of 8.43% appears at 440–560 °C, accompanied with a strong sharp exothermic peak at around 530 °C, which belongs to the decomposition and combustion of 3-pyridine carboxylic acid groups and [SiW12O40]4−. The total weight loss of compound 3 is about 18.43% (except the absorbed water weight loss), which agrees with the calculated value (14.24%) in the range of errors permitted. The TG–DTA curve of 4 is shown in Fig. S5d. It gives a total weight loss of 32.66% in the range of 25–800 °C. The weight loss is 14.25% in the range of 43–250 °C, with an endothermic peak at around 65 °C, which corresponds to the loss of absorbed water molecules. The last weight loss of 17.51% at 250–700 °C arises from the loss of structural water molecules, organic groups and decomposition of the polyoxoanions framework [VW12O40]3−, accompanied by endothermic and exothermic peaks at around 327, 525, 555, 606 °C. Above 700 °C the product of V2O5 start decompose to O2 and V2O4 accompanied by a weight loss of 0.9%. In the process the weight loss of compound 4 is 18.41%, compared with calculated one (13.5%) the difference is a bit big but within the error allowable range.
It is worth noting that the start decomposition temperatures of the four compounds are almost neck and neck at around 250 °C, while the complete decomposition temperature of these compounds is different. It is major that the stabilities of their heteropolyanions are obviously different, which in order of 2 > 4>1 > 3 (Fig. S5).
Cyclic Voltammetry
Two platinum electrodes were used respectively as the working electrode and the indicator electrode. The calomel electrode used as the reference electrode. Scanning voltage are from −1.2 to 0.2 V (for compound 1 and 4) and −0.8 to 0.2 V (for compound 2 and 3), and scanning speed is 20 mV s−1 with 1 mol L−1 NaCl solution as electrolyte. The cyclic voltammetry of 1 × 10−3 mol L−1 compound 1–4 solutions were measured in DMF-water (V: V = 1:1) system at room temperature after inlet pure N2 to expel dissolved oxygen.
Figure 4a–d show cyclic voltammograms of four compounds. 1, 2 and 3 show three pairs symmetrical redox peaks and 4 shows four pairs symmetrical redox peaks in the test conditions.
Compound 1 ([PW12O40]3− type) shows three pairs symmetrical redox peaks of I–I′, II–II′ and III–III′ at −1.028/−1.112, −0.772/−0.864 and −0.145/0.030 V, respectively, with mean peak potentials E1/2 = (Epa + Epc)/2 of −1.070, −0.809 and −0.258 V, and the separations of the Epc and Epa values ∆Ep = Epa − Epc are 84, 92, and 175 mV respectively. which are assigned to the redox process of W [27]. The redox peaks of I–I′ and II–II′ correspond to reversible redox processes while III–III′ is irreversible reaction based on ∆Ep symmetry and ipa/ipc of peaks.
For compound 2 ([PMo12O40]3− type), redox peaks are shown at −0.660/−0.747, −0.469/−0.547 and −0.153/−0.355 V, with E1/2 of −0.704, −0.508 and −0.254 V, and corresponding ∆Ep are 87, 78, and 202 mV, respectively, which are assigned the redox process of Mo [28]. Based on reversible criterion, I–I′ and II–II′ are quasi-reversible redox processes, while III–III′ is irreversible.
For compound 3 ([SiW12O40]4− type), redox peaks are at −0.641/−0.655, −0.463/−0.516 and −0.192/−0.303 V, with E1/2 of −0.648, −0.489 and −0.248 V, and ∆Ep are 14, 53, and 111 mV respectively, which are assigned to the redox wave of W [29, 30]. The redox waves of I–I′ and II–II′ are reversible redox processes based on the ∆Ep, while it’s quasi-reversible for III–III′.
For compound 4 ([VW12O40]3− type), four pairs symmetrical redox peaks of I–I′, II––II′, III–III′ and IV–IV′ appear at −1.036/−1.152, −0.847/−0.916, −0.653/−0.769 and −0.345/−0.394 V, with E1/2 of −1.094, −0.8815, −0.711 and −0.3695 V, respectively, corresponding to four one-electron redox waves [31]. The ∆Ep are 116, 69, 116, 49 mV, respectively, that I–I′ to III–III′ redox peaks undergo quasi-reversible redox processes while IV–IV′ is assigned to reversible. It is well known that in the same testing conditions the E1/2 values of the first one-electron waves are almost identical for the Keggin-type [XM12O40]n− (M = W or Mo) anions with identical ionic charge, and show a linear dependence on their ionic charge of [XMo12O40]n−or [XW12O40]n− [28, 31, 32]. It should be noted that the fourth one-electron wave of compound 4 is situated at around ca. −0.3695 V more negative than the corresponding waves of compound 1, 2 and 3, suggesting that the fourth one-electron wave of 4 cannot be ascribed to the reduction of W in the peripheral position, and the reduction of the central V atom may be responsible for the fourth wave [31], while the other three pairs peaks are redox processes of W.
According to the measured redox potential, the oxidability of compounds can be inferred in order of 3 > 2 > 1 > 4. Obviously, comparing the two isostructural compound 1 ([PW12O40]3− type) and 2 ([PMo12O40]3− type), indicating 2 is more effective oxidizing reagent that with more positive redox potential, which in agreement with literature report [33]. That cyclic voltammograms data can further provide theoretical basis for oxidation catalytic activity of compounds.
In addition, according to the literature [34], if E1/2 < −0.35 V, the reductive product heteropoly blues (HPBs) has strong reducing ability. The HPBs can be easily oxidized by O2 in the air to achieve regeneration of the catalyst. Compound 1–4 are almost all with the redox processes’ E1/2 < −0.35 V, indicating that their reduction products can be easily oxidized by O2 in the air, which is important for catalytic reaction.
Catalytic Properties
Blank experiments showed that the reaction substrate in a simulacrum of polluted air was hardly eliminated without compounds as the catalyst. From the results of the effluent gases after catalytic reaction, there is no new peak in the GC diagram, indicating no new organic compounds formed; lime saturated water formed precipitate and the color of the 0.2% PdCl2 solution did not change, indicating that there was CO2 but no CO. Products of the elimination of reaction substrate were CO2 and H2O.
Using grinding compounds 1–4 crystal powders as catalysts, the relationships between the elimination rate and temperature are shown in Fig. 5. When the initial concentration of methanol is 2.5 g m−3, flow velocity of 10 mL min−1 with the rise of temperature, elimination rate of methanol was improved successively. With the increase of temperature, the eliminate rate increases first, and reach higher elimination rates for methanol at 125 °C, that of 65% for 1, 85% for 2, 88% for 3, and 80% for 4, respectively, which correspond to the highest elimination rate of compounds 1, 2 and 4, but for 3 that of the highest 94% appears at 150 °C. After reaching the maximum elimination temperature, the elimination rates of methanol decrease, which may be that the methanol adsorption over the catalyst reduced at high temperature leading to the catalytic ability rapidly decline. Obviously, the maximum catalytic activity is in order of 3 > 2>4 > 1, which are ascribed to the differences of oxidizability, stability and adsorbability of the catalysts. It is well know that oxidation catalytic activity of compound is proportional to its oxidizing ability. The oxidability order of these four compounds is 3 > 2 > 1 > 4, the catalytic active should be consistent with that in theoretically, but inverted result are showed in compound 1 and 4, which may due to the stability of the catalytic activity of heteropolyanion. [VW12O40]3− of 4 is more stable than [PW12O40]3− of 1 according to TG–DTA data, in the relative high temperature, that compound 4 shows higher catalytic activity than that of 1.
In addition, it’s worth noting that at high temperature, the elimination rates of 1 and 2 decline rapidly, but for 3 and 4 that is slow, which may be largely influenced by the adsorbability. As for that compound 3 and 4 have more organic components than 1 and 2, that 3 and 4 have larger adsorbability to methanol than 1 and 2 at high temperature, this is also why 3 still exhibits good catalytic activity at high temperature and its highest elimination rate appears at 150 °C but 125 °C.
In order to compare catalytic performance of the H3PMoO40/H3PWO40 acids and the complexes,the pure parent H3PMoO40/H3PWO40 acids (which are commercial) as catalyst for oxidizing methanol have even been done in our group [35, 36]. Results indicate that pure parent H3PMoO40/H3PWO40 acids as the catalyst shows lower activity, poorer stability, and easier inactivation than its hybrid compound.
Conclusions
In this work, we have successfully assembled four 3D POM-based inorganic–organic hybrids from POM and 1H-1,2,4-Triazole or 2,3-Pyridinedicarboxylic acid under hydrothermal conditions. There are a number of hydrogen bonds and/or π–π stacking interactions in the compounds, which promote the forming of 3D novel network structure. Compound 1 and 2 are isostructural, both exhibit extended three-dimensional hole-net intersected channels via hydrogen bonding interactions. Compound 3 and 4 possess large-scale complicated structures, in which the 2,3-Pyridinedicarboxylic acid groups undergo decarboxylation reaction producing some groups [C6H6O2N]+, [CHO2]−, [C6O2NH4]−, [C5NH6]+ and [C6NO2H5]. And compound 4 shows a fancy disordered “α-Keggin” heteropolyanion [VW12O40]4−. In addition they show good oxidation catalytic activities toward methanol in a continuous-flow fixed-bed micro-reactor, when the initial concentration of methanol is 2.5 g m−3 in air and flow rate is 10 mL min−1, the corresponding elimination rates of methanol arrive 65% (125 °C) for 1, 85% (125 °C) for 2, 94% (150 °C) for 3, and 80% (125 °C) for 4, respectively.
References
R. Dessapt, M. Gabard, M. Bujoli-Doeuff, P. Deniard, and S. Jobic (2011). Inorg. Chem. 50, 8790–8796.
M. Ibrahim, Y. Lan, B. S. Bassil, et al. (2011). Hexadecacobalt(II)-containing polyoxometalate-based single-molecule magnet. Angew. Chem. Int. Ed. 50, 4708–4711.
S. Omwoma, C. T. Gore, Y. Ji, C. Hu, and Y. F. Song (2015). Coord. Chem. Rev. 286, 17–29.
A. Bijelic and A. Rompel (2015). Coord. Chem. Rev. 299, 22–38.
J. T. Rhule, C. L. Hill, D. A. Judd, et al. (1998). Chem. Rev. 98, 327–358.
J. Ettedgui, Y. Diskinposner, L. Weiner, et al. (2011). J. Am. Chem. Soc. 133, 188–190.
P. Gouzerh and A. Proust (1998). Chem. Rev. 98, 77–111.
L. Fu, H. Gao, M. Yan, et al. (2015). Small 11, 2938–2945.
X. J. Dui, W. B. Yang, X. Y. Wu, et al. (2015). Dalton Trans. 44, 9496–9505.
L. W. Han, J. X. Lin, and Q. Yin (2016). Cryst. Growth Des. 16, 1213–1217.
S. Roy, S. Sarkar, J. Pan, et al. (2016). Inorg. Chem. 55, 3364–3377.
Y. Ren, M. Wang, X. Chen, et al. (2015). Materials 8, 1545–1567.
P. G. Reddy, V. S. V. Satyanarayana, V. Dubey, et al. (2015). Inorg. Chem. Commun. 56, 65–68.
C. L. Teng, H. X. Xiao, Q. Cai, et al. (2016). J. Solid State Chem. 243, 146–153.
X. Zheng, L. Zhang, J. Li, et al. (2011). Chem. Commun. 47, 12325–12327.
Q. Deng, Y. L. Huang, Z. S. Peng, et al. (2013). J. Solid State Chem. 200, 60–69.
X. Li, Y. Chen, X. L. Chi, et al. (2015). Inorg. Chim. Acta 437, 159–166.
F. Grepioni, G. Cojazzi, S. M. Draper, N. Scully, and D. Braga (1998). Organometallics 17, 296–307.
J. Y. Niu, S. W. Zhang, and H. N. Chen (2011). Cryst. Growth Des. 11, 3769–3777.
B. X. Dong, J. Peng, N. H. Hu, et al. (2007). Inorg. Chem. 46, 5933–5941.
G.M. Sheldrick, SADABS, Germany, 1996
G.M. Sheldrick, SHELXS-97, Germany, 1997
C. H. Li, K. L. Huang, Y. N. Chi, X. Liu, Z. G. Han, L. Shen, and C. W. Hu (2009). Inorg. Chem. 48, 2010–2017.
S. L. Li, Y. Q. Lan, J. F. Ma, et al. (2008). Cryst. Growth Des. 8, 1610–1616.
Z. G. Han, Y. L. Zhao, J. Peng, H. Y. Ma, Q. Liu, E. B. Wang, and N. H. Hu (2004). J. Solid State Chem. 177, 4325–4331.
E.B. Wang, Introduction of POMs, Beijing, 1997
S. Himeno, M. Takamoto, and T. Ueda (1999). J. Electroanal. Chem. 465, 129–135.
M. Sadakane and E. Steckhan (1998). Chem. Rev. 98, 219–237.
M. T. Pope Heteropoly and isopoly oxometalates (Springer, New York, 1983).
S. J. Dong, X. D. Xi, and M. Tian (1995). J. Electroanal. Chem. 385, 227–233.
S. Himeno, M. Takamoto, A. Higuchi, et al. (2003). Inorg. Chim. Acta 348, 57–62.
K. Maeda, H. Katano, T. Osakai, S. Himeno, and A. Saito (1995). J. Electroanal. Chem. 389, 167–173.
T. He and J. Yao (2006). Prog. Mater. Sci. 51, 810–879.
Y. Dawei (1999). Petrochemical Universities 12, 11–13.
Y. Z. Han, et al. (2016). Synth. React Inorg. M 46, 351–364.
Y. Z. Han Research on synthesis, characterization and catalytic properties of keggin type polyoxometalates compounds based on pyrimidine and triazole (Hunan University of Science and Technology, Xiangtan, 2015).
Acknowledgements
This work was supported by Scientific Research Project of Hunan Province Education Department of China (Project No. 14C0459); Key Laboratory of Theoretical Organic Chemistry and Functional Molecules of Ministry of Education and the Organic Chemistry Key Subject of Hunan Province of China.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Teng, C., Han, Y., Xiao, H. et al. Hydrothermal Synthesis, Crystal Structure, and Catalytic Performance of Four Organic–Inorganic Hybrids Based on Polyoxometalates and O/N-Containing Groups. J Clust Sci 28, 2461–2475 (2017). https://doi.org/10.1007/s10876-017-1234-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-017-1234-9