Abstract
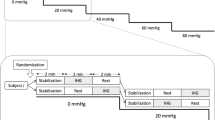
Transcranial Doppler ultrasonography (TCD) is used widely to evaluate dynamic cerebral autoregulation (dCA). However, the validity of TCD-determined dCA remains unknown because TCD is only capable of measuring blood velocity and thus only provides an index as opposed to true blood flow. To test the validity of TCD-determined dCA, in nine healthy subjects, dCA was evaluated by transfer function analysis (TFA) using cerebral blood flow (CBF) or TCD-measured cerebral blood velocity during a perturbation that induces reductions in TCD-determined dCA, lower body negative pressure (LBNP) at two different stages: LBNP − 15 mmHg and − 50 mmHg. Internal carotid artery blood flow (ICA Q) was assessed as an index of CBF using duplex Doppler ultrasound. The TFA low frequency (LF) normalized gain (ngain) calculated using ICA Q increased during LBNP at − 50 mmHg (LBNP50) from rest (P = 0.005) and LBNP at − 15 mmHg (LBNP15) (P = 0.015), indicating an impaired dCA. These responses were the same as those obtained using TCD-measured cerebral blood velocity (from rest and LBNP15; P = 0.001 and P = 0.015). In addition, the ICA Q-determined TFA LF ngain from rest to LBNP50 was significantly correlated with TCD-determined TFA LF ngain (r = 0.460, P = 0.016) despite a low intraclass correlation coefficient. Moreover, in the Bland–Altman analysis, the difference in the TFA LF ngains determined by blood flow and velocity was within the margin of error, indicating that the two measurement methods can be interpreted as equivalent. These findings suggest that TCD-determined dCA can be representative of actual dCA evaluated with CBF.





Similar content being viewed by others
Data availability
The data that supports the findings of this study are available within the article.
References
Hellström G, Fischer-Colbrie W, Wahlgren NG, Jogestrand T. Carotid artery blood flow and middle cerebral artery blood flow velocity during physical exercise. J Appl Physiol. 1996;81:413–8.
Sato K, Sadamoto T, Hirasawa A, Oue A, Subudhi AW, Miyazawa T, et al. Differential blood flow responses to CO2 in human internal and external carotid and vertebral arteries. J Physiol. 2012;590:3277–90.
Ainslie PN, Hoiland RL. Transcranial doppler ultrasound: Valid, invalid, or both? J Appl Physiol. 2014;117:1081–3.
Coverdale NS, Gati JS, Opalevych O, Perrotta A, Shoemaker JK. Cerebral blood flow velocity underestimates cerebral blood flow during modest hypercapnia and hypocapnia. J Appl Physiol. 2014;117:1090–6.
Coverdale NS, Lalande S, Perrotta A, Shoemaker JK. Heterogeneous patterns of vasoreactivity in the middle cerebral and internal carotid arteries. Am J Physiol - Hear Circ Physiol. 2015;308:H1030–8.
Verbree J, Bronzwaer ASGT, Ghariq E, Versluis MJ, Daemen MJAP, Van Buchem MA, et al. Assessment of middle cerebral artery diameter during hypocapnia and hypercapnia in humans using ultra-high-field MRI. J Appl Physiol. 2014;117:1084–9.
Claassen JAHR, Thijssen DHJ, Panerai RB, Faraci FM. Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol Rev. 2021;101:1487–559.
Brassard P, Labrecque L, Smirl JD, Tymko MM, Caldwell HG, Hoiland RL, et al. Losing the dogmatic view of cerebral autoregulation. Physiol Rep. 2021;9:1–11.
Sato K, Fisher JP, Seifert T, Overgaard M, Secher NH, Ogoh S. Blood flow in internal carotid and vertebral arteries during orthostatic stress. Exp Physiol. 2012;97:1272–80.
Ogoh S, Sørensen H, Hirasawa A, Sasaki H, Washio T, Hashimoto T, et al. Dynamic cerebral autoregulation is unrelated to decrease in external carotid artery blood flow during acute hypotension in healthy young men. Exp Physiol. 2016;101:1040–9.
Newell DW, Aaslid R, Lam A, Mayberg TS, Winn HR. Comparison of flow and velocity during dynamic autoregulation testing in humans. Stroke. 1994;25:793–7.
Ogoh S, Nakata H, Miyamoto T, Bailey DM, Shibasaki M. Dynamic cerebral autoregulation during cognitive task: effect of hypoxia. J Appl Physiol. 2018;124:1413–9.
Zhang R, Zuckerman JH, Levine BD. Deterioration of cerebral autoregulation during orthostatic stress: insights from the frequency domain. J Appl Physiol. 1998;85:1113–22.
Zhang R, Zuckerman JH, Pawelczyk JA, Levine BD. Effects of head-down-tilt bed rest on cerebral hemodynamics during orthostatic stress. J Appl Physiol. 1997;83:2139–45.
Ogoh S, Lericollais R, Hirasawa A, Sakai S, Normand H, Bailey DM. Regional redistribution of blood flow in the external and internal carotid arteries during acute hypotension. Am J Physiol Integr Comp Physiol. 2014;306:R747–51.
Nogueira RC, Saeed NP, Bor-Seng-Shu E, Teixeira MJ, Robinson TG, Panerai RB. The carotid artery as an alternative site for dynamic autoregulation measurement: an inter-observer reproducibility study. Med Eng Phys. 2016;38:690–4.
Chi NF, Ku HL, Wang CY, Liu Y, Chan L, Lin YC, et al. Dynamic cerebral autoregulation assessment using extracranial internal carotid artery Doppler ultrasonography. Ultrasound Med Biol. 2017;43:1307–13.
Ichikawa D, Miyazawa T, Horiuchi M, Kitama T, Fisher JP, Ogoh S. Relationship between aerobic endurance training and dynamic cerebral blood flow regulation in humans. Scand J Med Sci Sport. 2013;23:e320–9.
Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke. 2000;31:1672–8.
Verbree J, Bronzwaer AGT, van Buchem MA, Daemen MJAP, van Lieshout JJ, van Osch MJP. Middle cerebral artery diameter changes during rhythmic handgrip exercise in humans. J Cereb Blood Flow Metab. 2017;37:2921–7.
Al-Khazraji BK, Shoemaker LN, Gati JS, Szekeres T, Shoemaker JK. Reactivity of larger intracranial arteries using 7 T MRI in young adults. J Cereb Blood Flow Metab. 2019;39:1204–14.
Burma JS, Copeland P, Macaulay A, Khatra O, Wright AD, Smirl JD. Dynamic cerebral autoregulation across the cardiac cycle during 8 hr of recovery from acute exercise. Physiol Rep. 2020;8:1–15.
Washio T, Sasaki H, Ogoh S. Transcranial Doppler-determined change in posterior cerebral artery blood flow velocity does not reflect vertebral artery blood flow during exercise. Am J Physiol Circ Physiol. 2017;312:H827–31.
Burma JS, Copeland P, Macaulay A, Khatra O, Smirl JD. Comparison of diurnal variation, anatomical location, and biological sex within spontaneous and driven dynamic cerebral autoregulation measures. Physiol Rep. 2020;8:e14458.
Smirl JD, Hoffman K, Tzeng YC, Hansen A, Ainslie APN. Methodological comparison of active- and passive-driven oscillations in blood pressure; implications for the assessment of cerebral pressure-flow relationships. J Appl Physiol. 2015;119:487–501.
Brown CM, Dütsch M, Hecht MJ, Neundörfer B, Hilz MJ. Assessment of cerebrovascular and cardiovascular responses to lower body negative pressure as a test of cerebral autoregulation. J Neurol Sci. 2003;208:71–8.
Wieling W, Krediet CTP, Van Dijk N, Linzer M, Tschakovsky ME. Initial orthostatic hypotension: review of a forgotten condition. Clin Sci. 2007;112:157–65.
Sanders ML, Elting JWJ, Panerai RB, Aries M, Bor-Seng-Shu E, Caicedo A, et al. Dynamic cerebral autoregulation reproducibility is affected by physiological variability. Front Physiol. 2019;10:1–11.
Willie CK, Colino FL, Bailey DM, Tzeng YC, Binsted G, Jones LW, et al. Utility of transcranial Doppler ultrasound for the integrative assessment of cerebrovascular function. J Neurosci Methods. 2011;196:221–37.
Thomas KN, Lewis NCS, Hill BG, Ainslie PN. Technical recommendations for the use of carotid duplex ultrasound for the assessment of extracranial blood flow. Am J Physiol - Regul Integr Comp Physiol. 2015;309:R707–20.
Radparvar JR, Lim G, Chiem AT. Effect of insonation angle on peak systolic velocity variation. Am J Emerg Med. 2020;38:173–7.
Zhang R, Zuckerman JH, Iwasaki K, Wilson TE, Crandall CG, Levine BD. Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation. 2002;106:1814–20.
Claassen JAHR, Meel-Van Den Abeelen AS, Simpson DM, Panerai RB, Dorado AC, Mitsis GD, et al. Transfer function analysis of dynamic cerebral autoregulation: a white paper from the International Cerebral Autoregulation Research Network. J Cereb Blood Flow Metab. 2015;36:665–80.
Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol - Hear Circ Physiol. 1998;274:H233–41.
Ogoh S, Dalsgaard MK, Yoshiga CC, Dawson EA, Keller DM, Raven PB, et al. Dynamic cerebral autoregulation during exhaustive exercise in humans. Am J Physiol Circ Physiol. 2005;288:H1461–7.
Ogoh S, Fadel PJ, Zhang R, Selmer C, Jans Ø, Secher NH, et al. Middle cerebral artery flow velocity and pulse pressure during dynamic exercise in humans. Am J Physiol Circ Physiol. 2005;288:H1526–31.
Diehl RR, Linden D, Lücke D, Berlit P. Phase relationship between cerebral blood flow velocity and blood pressure a clinical test of autoregulation. Stroke. 1995;26:1801–4.
Ogoh S, Dalsgaard MK, Secher NH, Raven PB. Dynamic blood pressure control and middle cerebral artery mean blood velocity variability at rest and during exercise in humans. Acta Physiol. 2007;191:3–14.
Diehl RR, Linden D, Lücke D, Berlit P. Spontaneous blood pressure oscillations and cerebral autoregulation. Clin Auton Res. 1998;8:7–12.
Panagiotakos DB. The value of p value in biomedical research. Open Cardiovasc Med J. 2008;2:97–9.
Halsey LG. The reign of the p-value is over: What alternative analyses could we employ to fill the power vacuum? Biol Lett. 2019;15:20190174.
Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:1–12.
Cohen J. Statistical power analysis for the behavioral sciences. New York: Routledge Academic; 1988.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;8:307–10.
Smith KJ, Hoiland RL, Grove R, McKirdy H, Naylor L, Ainslie PN, et al. Matched increases in cerebral artery shear stress, irrespective of stimulus, induce similar changes in extra-cranial arterial diameter in humans. J Cereb Blood Flow Metab. 2019;39:849–58.
Poggesi A, Pasi M, Pescini F, Pantoni L, Inzitari D. Circulating biologic markers of endothelial dysfunction in cerebral small vessel disease: A review. J Cereb Blood Flow Metab. 2016;36:72–94.
Patel N, Panerai RB, Haunton V, Katsogridakis E, Saeed NP, Salinet A, et al. The Leicester cerebral haemodynamics database: normative values and the influence of age and sex. Physiol Meas. 2016;37:1485–98.
Labrecque L, Rahimaly K, Imhoff S, Paquette M, Le Blanc O, Malenfant S, et al. Dynamic cerebral autoregulation is attenuated in young fit women. Physiol Rep. 2019;7:1–12.
Claassen JAHR, Levine BD, Zhang R. Dynamic cerebral autoregulation during repeated squat-stand maneuvers. J Appl Physiol. 2009;106:153–60.
Acknowledgments
The authors appreciate the time and effort expended by the volunteer subjects and staffs. Also, the authors would like to thank Prof. Paul Fadel (University of Texas at Arlington) for his helpful scientific comments for improving this manuscript.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This study was in part supported by a Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology (Grant Number 15H003098).
Author information
Authors and Affiliations
Contributions
HW and SO conception and design of research; HW, TW, SS, AH, RS, SS, SO performed experiments; HW analyzed data; HW and SO interpreted results of experiments; HW prepared figures; HW, MB, SO drafted manuscript; all authors edited and revised manuscript; all authors approved final version of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest related to the subject matter or materials discussed in this article. All data of the present study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Ethical approval
This study was approved by the ethics committee of Kyorin University (Approval Number 723) in accordance with the Declaration of Helsinki.
Informed consent
All subjects provided written informed consent prior to participation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Watanabe, H., Washio, T., Saito, S. et al. Validity of transcranial Doppler ultrasonography-determined dynamic cerebral autoregulation estimated using transfer function analysis. J Clin Monit Comput 36, 1711–1721 (2022). https://doi.org/10.1007/s10877-022-00817-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-022-00817-1