Abstract
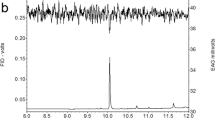
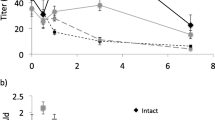
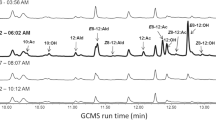
By differentially sampling the pheromone gland of females of the moth Heliothis virescens, we explored differences in pheromone on the surface, or outer distal layer(s) of the gland, and that located more proximally. For this, we used two sampling approaches, (i) a solid phase microextraction fiber rub followed by solvent extraction of residual pheromone (SPME rub/extract), and (ii) rapid solvent rinsing followed by solvent extraction of residual pheromone (rinse/extract). The SPME rub showed differences in component ratio between the dorsal and ventral gland surfaces. The rinse sampled a greater amount of pheromone than the SPME rub, sampling the whole gland surface as well as likely deeper into the gland. Compared to the other samplings, pheromone in the rinse was depleted in the minor component; consequently, the corresponding residual extract was highly enriched in the minor component. Further rinses of the gland yielded only small amounts of pheromone, with a similar component ratio as the first rinse, suggesting that the residual pheromone was less accessible and required extraction in solvent to be liberated. Sampling over the photoperiod showed that the more volatile minor component was depleted (relative to the major component) on the surface/outer cuticle over the period when females called. Together, these data suggest that the pheromone is stored, at least in part, on and in the gland cuticle and that distinct pools may be transported to different topographic regions. Females fed with a stable isotope tracer, incorporated label into pheromone in the gland very rapidly, with the labeled pheromone appearing on the gland surface ca. 1 min later.




Similar content being viewed by others
References
Allison JD, Cardé RT (eds) (2016) Pheromone communication in moths: evolution, behavior and application. University of Caifornia Press, Oakland
Ando T, Inomata S, Yamamoto M (2004) Lepidopteran sex pheromones. In: Schulz S (ed) The Chemistry of Pheromones and Other Semiochemicals I, vol 239. Topics in Current Chemistry. Springer Berlin / Heidelberg, pp 51–96. doi:https://doi.org/10.1007/b95449
Blomquist GJ, Jurenka R, Schal C, Tittiger C (2011) Pheromone production: biochemistry and molecular biology. In: Gilbert LI (ed) Insect Endocrinology. Academic Press, San Diego, pp 523–567
Chinkes DL, Aarsland A, Rosenblatt J, Wolfe RR (1996) Comparison of mass isotopomer dilution methods used to compute VLDL production in vivo. Am J Physiol Endocrinol Metab 271:E373–E383
Choi MY, Groot A, Jurenka RA (2005) Pheromone biosynthetic pathways in the moths Heliothis subflexa and Heliothis virescens. Arch Insect Biochem Physiol 59:53–58
Conner WE, Webster RP, Itagaki H (1985) Calling behaviour in arctiid moths: The effects of temperature and wind speed on the rhythmic exposure of the sex attractant gland. J Insect Physiol 31:815–820. https://doi.org/10.1016/0022-1910(85)90074-5
Cory HT, Daterman GE, Daves GD, Sower LL, Shepherd RF, Sanders CJ (1982) Chemistry and field evaluation of the sex pheromone of western spruce budworm. Choristoneura occidentalis, Freeman J Chem Ecol 8:339–350. https://doi.org/10.1007/bf00987782
Delisle J (1992) Age related changes in the calling behaviour and the attractiveness of obliquebanded leafroller virgin females, Choristoneura rosaceana, under different constant and fluctuating conditions. Entomologia experimentalis et applicata 63:55–62
Fang NB, Teal PEA, Tumlinson JH (1995) Characterization of oxidase(s) associated with the sex pheromone gland in Manduca sexta (L) females. Arch Insect Biochem Physiol 29:243–257
Fonagy A, Yokoyama N, Okano K, Tatsuki S, Maeda S, Matsumoto S (2000) Pheromone-producing cells in the silkmoth, Bombyx mori: identification and their morphological changes in response to pheromonotropic stimuli. J Insect Physiol 46:735–744
Foster S, Anderson K (2011) The use of mass isotopomer distribution analysis to quantify synthetic rates of sex pheromone in the moth Heliothis virescens. J Chem Ecol 37:1208–1210
Foster SP (2005) Lipid analysis of the sex pheromone gland of the moth Heliothis virescens. Arch Insect Biochem Physiol 59:80–90. https://doi.org/10.1002/arch.20058
Foster SP (2016) Toward a quantitative paradigm for sex pheromone production in moths. In: Allison JD, Cardé RT (eds) Pheromone communication in moths: evolution, behavior and application. University of California Press, Oakland, pp 113–126
Foster SP, Anderson KG (2015) Sex pheromones in mate assessment: analysis of nutrient cost of sex pheromone production by females of the moth Heliothis virescens. J Exp Biol 218:1252–1258. https://doi.org/10.1242/jeb.119883
Foster SP, Anderson KG, Casas J (2017) Sex pheromone in the moth Heliothis virescens is produced as a mixture of two pools: de novo and via precursor storage in glycerolipids. Insect Biochem Mol Biol 87:26–34. https://doi.org/10.1016/j.ibmb.2017.06.004
Frérot B, Malosse C, Cain A-H (1997) Solid-phase microextraction (SPME): A new tool in pheromone identification in lepidoptera. J High Resolut Chromatogr 20:340–342. https://doi.org/10.1002/jhrc.1240200609
Groot AT (2014) Circadian rhythms of sexual activities in moths: a review. Front Ecol Evol 2. https://doi.org/10.3389/fevo.2014.00043
Hagström ÅK, Walther A, Wendland J, Löfstedt C (2013) Subcellular localization of the fatty acyl reductase involved in pheromone biosynthesis in the tobacco budworm, Heliothis virescens (Noctuidae: Lepidoptera). Insect Biochem Mol Biol 43:510–521. https://doi.org/10.1016/j.ibmb.2013.03.006
Heath RR, McLaughlin JR, Proshold F, Teal PEA (1991) Periodicity of female sex pheromone titer and release in Heliothis subflexa and H. virescens (Lepidoptera: Noctuidae). Ann Entomol Soc Am 84:182–189
Hellerstein MK, Neese RA (1999) Mass isotopomer distribution analysis at eight years: theoretical, analytic, and experimental considerations. Am J Physiol Endocrinol Metab 276:E1146–E1170
Jurenka R (2017) Regulation of pheromone biosynthesis in moths. Current Opinion in Insect Science 24:29–35. https://doi.org/10.1016/j.cois.2017.09.002
Jurenka RA, Roelofs WL (1989) Characterization of the acetyltransferase used in pheromone biosynthesis in moths: specificity for the Z isomer in Tortricidae. Insect Biochem 19:639–644
Klun JA et al (1980) Sex pheromone chemistry of the female tobacco budworm moth, Heliothis virescens. J Chem Ecol 6:177–184
Lievers R, Groot AT (2016) Disposable Polydimethylsiloxane (PDMS)-Coated Fused Silica Optical Fibers for Sampling Pheromones of Moths. PLoS One 11:e0161138. https://doi.org/10.1371/journal.pone.0161138
Ma PWK, Ramaswamy SB (2003) Biology and ultrastructure of sex pheromone-producing tissue. In: Blomquist GJ, Vogt RC (eds) Insect pheromone biochemsitry and molecular biology. Elsevier Academic Press, London, pp 19–51
Pawliszyn J (1997) Solid phase microextraction: theory and practice. Wiley-VCH, New York
Raina AK, Wergin WP, Murphy CA, Erbe EF (2000) Structural organization of the sex pheromone gland in Helicoverpa zea in relation to pheromone production and release. Arthropod Structure and Development 29:343–353
Ramaswamy SB (1990) Periodicity of oviposition, feeding, and calling by mated female Heliothis virescens in a field cage. J Insect Behav 3:417–427. https://doi.org/10.1007/BF01052118
Roelofs WL, Hill AS, Cardé RT, Baker TC (1974) Two sex pheromone components of the tobacco budworm moth, Heliothis virescens. Life Sci 14:1555–1562. https://doi.org/10.1016/0024-3205(74)90166-0
Teal PEA, Tumlinson JH (1988) Properties of cuticular oxidases used for sex pheromone biosynthesis by Heliothis zea. J Chem Ecol 14:2131–2145
Zhu J, Löfstedt C, Bengtsson BO (1996) Genetic variation in the strongly canalized sex pheromone communication system of the European corn borer, Ostrinia nubilalis Hübner (Lepidoptera, Pyralidae). Genetics 144:757–766
Acknowledgments
This work was funded in part by a United States Department of Agriculture Hatch Project ND02388 (to SPF). We thank the United States Department of Agriculture–National Institute of Food and Agriculture for an Instrument Grant, 2015-07238 (to SPF), contributing to the purchase of the GC/MS system. We are grateful to Professor Jérôme Casas for comments on an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Foster, S.P., Anderson, K.G. Differential Pheromone Sampling of the Gland of Female Heliothis Virescens Moths Reveals Glandular Differences in Composition and Quantity. J Chem Ecol 44, 452–462 (2018). https://doi.org/10.1007/s10886-018-0954-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-018-0954-0