Abstract
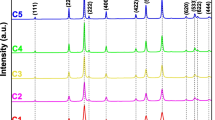
Nickel oxide (NiO) nanoparticles were formed using the chemical precipitation method. The effect of the calcination process on the structural parameters, optical bandgap, and photocatalytic performance was investigated. The structural characteristics were carried out using X-ray diffraction (XRD), Fourier transforms infrared spectroscopy (FTIR), and scanning electron microscope (SEM). The XRD analysis reveals that the formed NiO crystallized in an fcc crystal structure and the calcination process influences the crystallite size, microstrain, dislocation density, and average surface area. For example, the smallest and largest particle sizes (19.13 nm and 27.63 nm) were achieved for the samples prepared at 800 °C for 4 h and 900 °C for 2 h, respectively. Based on the diffuse reflectance spectroscopy analysis, the energy bandgap has the lowest values (3.33 eV) for the prepared NiO that calcinated at 800 °C for 2 h compared with other samples. The formation of a Ni–O stretching vibration mode is revealed by FTIR, and the broadness of the absorption band confirms that the NiO samples are nanocrystals. The morphology of the prepared NiO reveals the formation of spherical nanoparticles for NiO calcinated at 700 °C, while dodecahedron-like shapes were observed for NiO calcinated at 800 and 900 °C. The photocatalytic performance of NiO nanoparticles as catalysts for the degradation of indigo carmine dye was investigated under ultraviolet–visible irradiation up to 3 h. The best degradation efficiency was found to be 76% for NiO calcinated at 800 °C for 4 h, which belonged to the smallest crystallite size of 19.13 nm, and the highest surface area of 47.02 m2 g−1. The superior and excellent performance of this sample compared to other samples was confirmed by achieving the highest reaction rate constant (4.51 × 10−3 min−1). The proposed photodegradation mechanism shows the importance of increasing the time required for the recombination process between the positive holes and the excited electrons, which is the best possible when using the optimum photocatalyst sample that was prepared at 800 °C for 4 h.










Similar content being viewed by others
References
A.A. Balhaddad et al., Metal oxide nanoparticles and nanotubes: ultrasmall nanostructures to engineer antibacterial and improved dental adhesives and composites. Bioengineering 8(10), 146 (2021)
S.A.A. Shukor et al., Metal oxide and activated carbon as photocatalyst for waste water treatment. IOP Conf. Ser.: Mater. Sci. Eng. 557(1), 012066 (2019)
S. Sekar, D.Y. Kim, S. Lee, Excellent oxygen evolution reaction of activated carbon-anchored NiO nanotablets prepared by green routes. Nanomaterials 10, 1382 (2020)
T. Linda, S. Muthupoongodi, X.S. Shajan, S. Balakumar, Fabrication and characterization of chitosan templated CdO/NiO nanocomposite for dye degradation. Optik 127, 8287–8293 (2016)
B.J. Rani, G. Ravi, R. Yuvakkumar, S. Ravichandran, F. Ameen, A. Al-Sabri, Efficient, highly stable Zn-doped NiO nanocluster electrocatalysts for electrochemical water splitting applications. J. Sol–Gel Sci. Technol. 89, 500–510 (2019)
H.L.S. Santos, P.G. Corradini, M. Medina, J.A. Dias, L.H. Mascaro, NiMo–NiCu inexpensive composite with high activity for hydrogen evolution reaction. ACS Appl. Mater. Interfaces 12, 17492–17501 (2020)
V. Selvanathan, M.H. Ruslan, M. Aminuzzaman, G. Muhammad, N. Amin, K. Sopian, M. Akhtaruzzaman, Resorcinol formaldehyde (RF) as a novel plasticizer for starch-based solid biopolymer electrolyte. Polymers 12, 2170 (2020)
M. Akbayrak, A.M. Önal, Metal oxides supported cobalt nanoparticles: active electrocatalysts for oxygen evolution reaction. Electrochim. Acta 393, 139053 (2021)
Z.T. Althagafi et al., Differential electroanalysis of dopamine in the presence of a large excess of ascorbic acid at a nickel oxide nanoparticle-modified glassy carbon electrode. J. Sens. (2020). https://doi.org/10.1155/2020/8873930
R. Vinoth, P. Karthik, K. Devan, B. Neppolian, M. Ashokkumar, TiO2–NiO p–n nanocomposite with enhanced sonophotocatalytic activity under diffused sunlight. Ultrason. Sonochem. 35, 655–663 (2017)
Q. Li, L.-S. Wang, B.-Y. Hu, C. Yang, L. Zhou, L. Zhang, Preparation and characterization of NiO nanoparticles through calcination of malate gel. Mater. Lett. 61, 1615–1618 (2007)
X. Xin, Z. Lü, B. Zhou et al., Effect of synthesis conditions on the performance of weakly agglomerated nanocrystalline NiO. J. Alloys Compd. 427, 251–255 (2007)
Y. Wu, Y. He, T. Wu, T. Chen, W. Weng, H. Wan, Influence of some parameters on the synthesis of nanosized NiO material by modified sol-gel method. Mater. Lett. 61, 3174–3178 (2007)
M.I. Awad et al., Nickel oxide nanoparticles modified gold electrode for fractional determination of dopamine and ascorbic acid. J. Anal. Chem. 73(12), 1188–1194 (2018)
A. Bafekry et al., Investigation of vacancy defects and substitutional doping in AlSb monolayer with double layer honeycomb structure: a first-principles calculation. J. Phys.: Condens. Matter 34(6), 065701 (2021)
A. Bafekry et al., Tunable electronic properties of porous graphitic carbon nitride (C6N7) monolayer by atomic doping and embedding: a first-principle study. Appl. Surf. Sci. 583, 152270 (2022)
A. Bafekry et al., Band-gap engineering, magnetic behavior and Dirac-semimetal character in the MoSi2N4 nanoribbon with armchair and zigzag edges. J. Phys. D 55(3), 035301 (2021)
A. Bafekry et al., Tunable electronic and magnetic properties of MoSi2N4 monolayer via vacancy defects, atomic adsorption and atomic doping. Appl. Surf. Sci. 559, 149862 (2021)
M. El-Kemary, N. Nagy, I. El-Mehasseb, Nickel oxide nanoparticles: synthesis and spectral studies of interactions with glucose. Mater. Sci. Semicond. Process. 16, 1747–1752 (2013)
F. Davar, Z. Fereshteha, M. Salavati-Niasari, Nanoparticles Ni and NiO: synthesis, characterization and magnetic properties. J. Alloys Compd. 476, 797–801 (2009)
H.M. Mohaideen, S.S. Fareed, B. Natarajan, Role of calcination temperatures on the structural and optical properties of NiO nanoparticle. Surf. Rev. Lett. 26, 1950043 (2019)
R.B. da Silva, R.A. Pinto, J.M. Soares, A. Franco Jr., M.A. Correa, F. Bohn, J.A.P. da Costa, Effect of the synthesis method and calcination temperature on the formation of Ni–NiO nanocomposites. J. Sol–Gel Sci. Technol. 91(2), 286–294 (2019)
M.R. Aravind, C. Kalaiselvi, B. Revathi, A.N. Grace, S. Pitchaimuthu, S. Suresh, V. Sindhu, N.K. Chandar, Influence of various concentrations of cetyltrimethylammonium bromide on the properties of nickel oxide nanoparticles for supercapacitor application. NANO 16(12), 2150138 (2021)
A.A. Ezhilarasi, J.J. Vijaya, K. Kaviy, L.J. Kennedy, R.J. Ramalingam, H.A. Al-Lohedan, Green synthesis of NiO nanoparticles using Aegle marmelos leaf extract for the evaluation of in-vitro cytotoxicity, antibacterial and photocatalytic properties. J. Photochem. Photobiol. B 180, 39–50 (2018)
T. Zahra, K.S. Ahmad, Structural, optical and electrochemical studies of organo-templated wet synthesis of cubic shaped nickel oxide nanoparticles. Optik 205, 164241 (2020)
K. Baranwal, L.M. Dwivedi, Shehala, V. Singh, Guar gum mediated synthesis of NiO nanoparticles: an efficient catalyst for reduction of nitroarenes with sodium borohydride. Int. J. Biol. Macromol. 120, 2431–2441 (2018)
M. Bhagat et al., Green synthesis of silver nanoparticles using aqueous extract of Rosa brunonii Lindl and their morphological, biological and photocatalytic characterizations. J. Inorg. Organomet. Polym Mater. 29(3), 1039–1047 (2019)
Prerna et al., Morphological and optical characterization of sol–gel synthesized Ni-doped ZnO nanoparticles. Integr. Ferroelectr. 205(1), 1–13 (2020)
A. Singh et al., Promising photocatalytic degradation of methyl orange dye via sol-gel synthesized Ag–CdS@Pr-TiO2 core/shell nanoparticles. Physica B 616, 413121 (2021)
A. Ahmed et al., Investigating the thermographical effect on optical properties of Eu doped Y2O3:TiO2 nanocomposite synthesized via sol–gel method. Solid State Sci. 116, 106617 (2021)
B. Singh et al., Electrochemical sensing and photocatalytic degradation of 2,4-dinitrophenol via bismuth(III) oxide nanowires. J. Mol. Struct. 1255, 132379 (2022)
B. Singh et al., Effect of Pd concentration on the structural, morphological and photodiode properties of TiO2 nanoparticles. J. Mater. Sci.: Mater. Electron. 31(1), 65–74 (2020)
R. Packiaraj, K. Mahendraprabhu, P. Devendran, N. Nallamuthu, B. Palanivel, K.S. Venkatesh, R. Karuppannan, Electrochemical performances of ZnO–NiO–CuO mixed metal oxides as smart electrode material for solid-state asymmetric device fabrication. Energy Fuels (2021). https://doi.org/10.1021/acs.energyfuels.1c02703
E.F. Abo Zeid, A.M. Nassar, M.A. Hussein, M.M. Alam, A.M. Asiri, H.H. Hegazy, M.M. Rahman, Mixed oxides CuO–NiO fabricated for selective detection of 2-aminophenol by electrochemical approach. J. Mater. Res. Technol. 9(2), 1457–1467 (2020)
A.M. Ali, R. Najmy, Structural, optical and photocatalytic properties of NiO–SiO2 nanocomposites prepared by sol–gel technique. Catal. Today 208, 2–6 (2013)
J. Ahmad, K. Majid, M.A. Dar, Controlled synthesis of p-type NiO/n-type GO nanocomposite with enhanced photocatalytic activity and study of temperature effect on the photocatalytic activity of the nanocomposite. Appl. Surf. Sci. 457, 417–426 (2018)
E.F. Abo Zeid, I.A. Ibrahem, A.A. Massad, W.A.A. Mohamed, The effect of CdO content on the crystal structure, surface morphology, optical properties and photocatalytic efficiency of p-NiO/n-CdO nanocomposite. Results Phys. 12, 562–570 (2019)
M. Junaid, M.M. Shah, A. Noor, M. Javed, Hydrothermal preparation of iron doped nickel oxide for electrochemical sensing of urea. Chem. Phys. Impact 3, 100052–100059 (2021)
J. Al Boukhair, A. Khalaf, R.S. Hassan, R. Awad, Structural, optical and magnetic properties of pure and rare earth-doped NiO nanoparticles. Appl. Phys. A 126, 1–13 (2020)
S. Layek, H.C. Verma, Room temperature ferromagnetism in Mn-doped NiO nanoparticles. J. Magn. Magn. Mater. 397, 73–78 (2016)
N.D. Cuong, T.D. Tran, Q.T. Nguyen, H. Van Minh Hai, T.T. Hoa, D.T. Quang, W. Klysubun, P.D. Tran, Highly porous Co-doped NiO nanorods: facile hydrothermal synthesis and electrocatalytic oxygen evolution properties. R. Soc. Open Sci. 8(9), 202352 (2021)
L.G. Teoh, K.-D. Li, Synthesis and characterization of NiO nanoparticles by sol–gel method. Mater. Trans. 53(12), 2135–2140 (2012)
K. Varunkumar, R. Hussain, G. Hegde, A.S. Ethiraj, Effect of calcination temperature on Cu doped NiO nanoparticles prepared via wet-chemical method: structural, optical and morphological studies. Mater. Sci. Semicond. Process. 66, 149–156 (2017)
A.L. Patterson, The Scherrer formula for X-ray particle size determination. Phys. Rev. 56(10), 978 (1939)
M. Vautier, C. Guillard, J.M. Herrmann, Photocatalytic degradation of dyes in water: case study of indigo and of indigo carmine. J. Catal. 201(1), 46–59 (2001)
A.K. Subramani et al., Photocatalytic degradation of indigo carmine dye using TiO2 impregnated activated carbon. Bull. Mater. Sci. 30(1), 37–41 (2007)
A.G.S. Prado et al., Nb2O5 as efficient and recyclable photocatalyst for indigo carmine degradation. Appl. Catal. B 82(3–4), 219–224 (2008)
R. Abdel-Aziz, M.A. Ahmed, M.F. Abdel-Messih, A novel UV and visible light driven photocatalyst AgIO4/ZnO nanoparticles with highly enhanced photocatalytic performance for removal of rhodamine B and indigo carmine dyes. J. Photochem. Photobiol. A 389, 112245 (2020)
M.S. Alshatwi, H.A. Alburaih, S.S. Alghamdi, D.A. Alfadhil, J.A. Alshehri, F.A. Aljamaan, Iron-doped nickel oxide nanoparticles synthesis and analyzing different properties. Adv. Sci. Technol. Eng. Syst. J. 6(1), 1422–1426 (2021)
Y.T. Prabhu et al., X-ray analysis by Williamson–Hall and size-strain plot methods of ZnO nanoparticles with fuel variation. World J. Nano Sci. Eng. (2014). https://doi.org/10.4236/wjnse.2014.41004
K.B. Baharudin, N. Abdullah, D. Derawi, Effect of calcination temperature on the physicochemical properties of zinc oxide nanoparticles synthesized by coprecipitation. Mater. Res. Express 5(12), 125018 (2018)
T. Thanit, C. Kongmark, Effect of calcination temperature on structural and optical properties of MAl2O4 (M= Ni, Cu, Zn) aluminate spinel nanoparticles. J. Adv. Ceram. 8(3), 352–366 (2019)
H. Qiao et al., Preparation and characterization of NiO nanoparticles by anodic arc plasma method. J. Nanomater. (2009). https://doi.org/10.1155/2009/795928
S. Sankar et al., Gel growth of α and γ glycine and their characterization. J. Cryst. Growth 312(19), 2729–2733 (2010)
A.I. Mitsionis, T.C. Vaimakis, C.C. Trapalis, The effect of citric acid on the sintering of calcium phosphate bioceramics. Ceram. Int. 36(2), 623–634 (2010)
Y. Wang et al., Preparation of NiO nanoparticles and their catalytic activity in the thermal decomposition of ammonium perchlorate. Thermochim. Acta 437(1–2), 106–109 (2005)
A.A. Christy, O.M. Kvalheim, R.A. Velapoldi, Quantitative analysis in diffuse reflectance spectrometry: a modified Kubelka–Munk equation. Vib. Spectrosc. 9(1), 19–27 (1995)
S. Sivakumar, N.A. Mala, Influence of variant temperatures in optical, magnetic properties of NiO nanoparticles and its supercapacitor applications via precipitation method. Asian J. Chem. 33(8), 1783–1790 (2021)
K. Karthik, S. Dhanuskodi, C. Gobinath, S. Prabukumar, S. Sivaramakrishnan, Multifunctional properties of microwave assisted CdO–NiO–ZnO mixed metal oxide nanocomposite: enhanced photocatalytic and antibacterial activities. J. Mater. Sci.: Mater. Electron. 29(7), 5459–5471 (2018)
N.M. Al-Hada et al., Thermal calcination-based production of SnO2 nanopowder: an analysis of SnO2 nanoparticle characteristics and antibacterial activities. Nanomaterials 8(4), 250 (2018)
S. Dwivedi et al., Calcination temperature reflected structural, optical and magnetic properties of nickel oxide. Magnetism 2, 45–55 (2022)
R.B. Kale, C.D. Lokhande, Influence of air annealing on the structural, optical and electrical properties of chemically deposited CdSe nano-crystallites. Appl. Surf. Sci. 223(4), 343–351 (2004)
O.I. Omotunde, A.E. Okoronkwo, A.F. Aiyesanmi, E. Gurgur, Photocatalytic behavior of mixed oxide NiO/PdO nanoparticles toward degradation of methyl red in water. J. Photochem. Photobiol. A 365, 145–150 (2018)
J. Feng, Y. Ding, Y. Guo, X. Li, W. Li, Calcination temperature effect on the adsorption and hydrogenated dissociation of CO2 over the NiO/MgO catalyst. Fuel 109, 110–115 (2013)
S. Samaneh, A. Nezamzadeh-Ejhieh, A p–n junction NiO–CdS nanoparticles with enhanced photocatalytic activity: a response surface methodology study. J. Mol. Liq. 257, 173–183 (2018)
K.V. Kumar, K. Porkodi, F. Rocha, Langmuir–Hinshelwood kinetics—a theoretical study. Catal. Commun. 9(1), 82–84 (2008)
K. Kannan et al., Structural studies of bio-mediated NiO nanoparticles for photocatalytic and antibacterial activities. Inorg. Chem. Commun. 113, 107755 (2020)
S.A. Bhat et al., Photocatalytic degradation of carcinogenic Congo red dye in aqueous solution, antioxidant activity and bactericidal effect of NiO nanoparticles. J. Iran. Chem. Soc. 17(1), 215–227 (2020)
A.A. Barzinjy et al., Green and eco-friendly synthesis of nickel oxide nanoparticles and its photocatalytic activity for methyl orange degradation. J. Mater. Sci.: Mater. Electron. 31, 11303–11316 (2020)
S. Saffarzadeh, G. Nabiyouni, F. Heidary, A short time microwave method for synthesis of magnetic NiFe2O4/NiO nanocomposites as a clean technology in photocatalytic degradation of water pollutants. J. Mater. Sci.: Mater. Electron. 30(9), 8171–8181 (2019)
Acknowledgements
The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work by Grant Code: (22UQU4250045DSR03).
Funding
This work was supported by Umm Al-Qura University [Grant No. 22UQU4250045DSR03].
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abd-Elnaiem, A.M., Hakamy, A., Ibrahem, I.A. et al. Thermal-Induced Effects on the Structural and Photocatalytic Properties of Nickel Oxide Nanoparticles for Indigo Carmine Dye Removal. J Inorg Organomet Polym 32, 2209–2220 (2022). https://doi.org/10.1007/s10904-022-02277-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-022-02277-1