Abstract
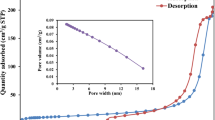
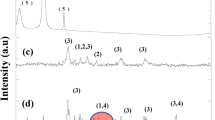
Hybrid crosslinked chitosan-epichlorohydrin/TiO2 nanocomposite (CTS-ECH/TNC) was synthesized as an inorganic–organic hybrid nanocomposite bioadsorbent for the removal of reactive red 120 (RR120) dye from aqueous environment. Various analytical techniques were utilized to investigate the surface area, surface morphology, martial crystallinity, elemental composition, amino group content, and fundamental functional group of CTS-ECH/TNC. The impact of key adsorption parameters such as adsorbent dosage (0.02–1.2 g), initial RR120 dye concentration (30–400 mg/L), solution pH (3–12), contact time (0–300 min), and temperature (303–323 K) were explored by batch adsorption process. The adsorption data were well illustrated by pseudo-second order (PSO) kinetic and Langmuir isotherm model. The adsorption process was also found to be a spontaneous and endothermic in nature as indicated by thermodynamic study. The maximum adsorption capacity of CTS-ECH/TNC for RR120 dye was recorded to be 210 mg/g at 303 K. Various types of interactions such as electrostatic attraction, n-π stacking, and H-bonding were responsible for adsorbing RR120 dye molecules on the surface of CTS-ECH/TNC as investigated by tailored adsorption mechanism. Thus, this work introduces CTS-ECH/TNC as promising hybrid biosorbent for the removal of RR120 dye as model of reactive azo dyes from aqueous environment.










Similar content being viewed by others
References
Rashid RA, Ishak MAM, Mohamed K (2018) Adsorptive removal of methylene blue by commercial coconut shell activated carbon. Sci Lett 12(1):77–101
Wang H, Zhong Y, Yu H, Aprea P, Hao S (2019) High-efficiency adsorption for acid dyes over CeO2· xH2O synthesized by a facile method. J Alloys Compd 776:96–104
Ziane S, Bessaha F, Marouf-Khelifa K, Khelifa A (2018) Single and binary adsorption of reactive black 5 and Congo red on modified dolomite: performance and mechanism. J Mol Liq 249:1245–1253
Değermenci GD, Değermenci N, Ayvaoğlu V, Durmaz E, Çakır D, Akan E (2019) Adsorption of reactive dyes on lignocellulosic waste: characterization, equilibrium, kinetic and thermodynamic studies. J Clean Prod 225:1220–1229
Tan KB, Vakili M, Horri BA, Poh PE, Abdullah AZ, Salamatinia B (2015) Adsorption of dyes by nanomaterials: recent developments and adsorption mechanisms. Sep Purif Technol 150:229–242
Konicki W, Aleksandrzak M, Moszyński D, Mijowska E (2017) Adsorption of anionic azo-dyes from aqueous solutions onto graphene oxide: equilibrium, kinetic and thermodynamic studies. J Colloid Interface Sci 496:188–200
Zaheer Z, Aisha AA, Aazam ES (2019) Adsorption of methyl red on biogenic Ag@ Fenanocomposite adsorbent: Isotherms, kinetics and mechanisms. J Mol Liq 283:287–298
Ghosh K, Harms K, Franconetti A, Frontera A, Chattopadhyay S (2019) A triple alkoxo bridged dinuclear cobalt (III) complex mimicking phosphatase and showing ability to degrade organic dye contaminants by photocatalysis. J. Organomet Chem 883:52–64
Nidheesh PV, Zhou M, Oturan MA (2018) An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes. Chemosphere 197:210–227
Beluci NDCL, Mateus GAP, Miyashiro CS, Homem NC, Gomes RG, Fagundes-Klen MR, Vieira AMS (2019) Hybrid treatment of coagulation/flocculation process followed by ultrafiltration in TiO2-modified membranes to improve the removal of reactive black 5 dye. Sci Total Environ 664:222–229
Abdulhameed AS, Mohammad AT, Jawad AH (2019) Modeling and mechanism of reactive orange 16 dye adsorption by chitosan-glyoxal/ TiO2 nanocomposite: application of response surface methodology. Desalin Water Treat 164:346–360
Jawad AH, Nawi MA, Mohamed MH, Wilson LD (2017) Oxidation of chitosan in solution by photocatalysis and product characterization. J Polym Environ 25:828–835
Wu J, Cheng X, Yang G (2019) Preparation of nanochitin-contained magnetic chitosan microfibers via continuous injection gelation method for removal of Ni (II) ion from aqueous solution. Int J Biol Macromol 125:404–413
Naskar S, Sharma S, Koutsu K (2018) Chitosan-based nanoparticles: an overview of biomedical applications and its preparation. J Drug Deliv Sci Technol 49:66–81
Jawad AH, Nawi MA (2012) Characterizations of the photocatalytically-oxidized cross-linked chitosan-glutaraldehyde and its application as a sub-layer in the TiO2/CS-GLA bilayer photocatalyst system. J Polym Environ 20:817–829
Vakili M, Rafatullah M, Salamatinia B, Abdullah AZ, Ibrahim MH, Tan KB, Amouzgar P (2014) Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: a review. Carbohydr Polym 113:115–130
Abdulhameed AS, Mohammad AT, Jawad AH (2019) Application of response surface methodology for enhanced synthesis of chitosan tripolyphosphate/TiO2 nanocomposite and adsorption of reactive orange 16 dye. J Clean Prod 232:43–56
Bilal M, Jing Z, Zhao Y, Iqbal HM (2019) Immobilization of fungal laccase on glutaraldehyde cross-linked chitosan beads and its bio-catalytic potential to degrade bisphenol A. Biocatal Agric Biotechnol 19:101174
Igberase E, Osifo PO (2019) Application of trimethylamine grafted on glyoxal cross-linked chitosan composite for the effective removal of metal ions in batch system. Int J Biol Macromol 134:1145–1155
Jawad AH, Mamat NH, Hameed BH, Ismail K (2019) Biofilm of cross-linked Chitosan-Ethylene Glycol Diglycidyl Ether for removal of Reactive Red 120 and Methyl Orange: adsorption and mechanism studies. J Environ Chem Eng 7:102965
Gutha Y, Zhang Y, Zhang W, Jiao X (2017) Magnetic-epichlorohydrin crosslinked chitosan schiff’s base (m-ECCSB) as a novel adsorbent for the removal of Cu (II) ions from aqueous environment. Int J Biol Macromol 97:85–98
Marrakchi F, Khanday WA, Asif M, Hameed BH (2016) Cross-linked chitosan/sepiolite composite for the adsorption of methylene blue and reactive orange 16. Int J Biol Macromol 93:1231–1239
Yan Y, Yuvaraja G, Liu C, Kong L, Guo K, Reddy GM, Zyryanov GV (2018) Removal of Pb (II) ions from aqueous media using epichlorohydrin crosslinked chitosan Schiff's base@ Fe3O4 (ECCSB@ Fe3O4). Int J Biol Macromol 117:1305–1313
Mohammad AT, Abdulhameed AS, Jawad AH (2019) Box-Behnken design to optimize the synthesis of new crosslinked chitosan-glyoxal/TiO2 nanocomposite: Methyl orange adsorption and mechanism studies. Int J Biol Macromol 129:98–109
Siripatrawan U, Kaewklin P (2018) Fabrication and characterization of chitosan-titanium dioxide nanocomposite film as ethylene scavenging and antimicrobial active food packaging. Food Hydrocoll 84:125–134
Cui HF, Wu WW, Li MM, Song X, Lv Y, Zhang TT (2018) A highly stable acetylcholinesterase biosensor based on chitosan-TiO2-graphene nanocomposites for detection of organophosphate pesticides. Biosens Bioelectron 99:223–229
Qu L, Chen G, Dong S, Huo Y, Yin Z, Li S, Chen Y (2019) Improved mechanical and antimicrobial properties of zein/chitosan films by adding highly dispersed nano-TiO2. Ind Crops Prod 130:450–458
Jawad AH, Nawi MA (2012) Oxidation of crosslinked chitosan-epichlorohydrine film and its application with TiO2 for phenol removal. Carbohydr Polym 90:87–94
Vieira RS, Beppu MM (2006) Interaction of natural and crosslinked chitosan membranes with Hg (II) ions. Coll Surf A 279:196–207
Dalvand A, Nabizadeh R, Ganjali MR, Khoobi M, Nazmara S, Mahvi AH (2016) Modeling of Reactive Blue 19 azo dye removal from colored textile wastewater using L-arginine-functionalized Fe3O4 nanoparticles: Optimization, reusability, kinetic and equilibrium studies. J Magn Magn Mater 404:179–189
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Frenudlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–471
Temkin MI (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochim URSS 12:327–356
Dubinin M (1960) The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem Rev 60:235–241
Weber TW, Chakravorti RK (1974) Pore and solid diffusion models for fixed-bed adsorbers. AIChE J 20:228–238
Sing KS (1985) Reporting physisorption data for gas, solid systems with special reference to the determination of surface area and porosity, (Recommendations 1984). Pure Appl Chem 57(1985):603–619
Maity J, Ray SK (2018) Chitosan based nano composite adsorbent—synthesis, characterization and application for adsorption of binary mixtures of Pb (II) and Cd (II) from water. Carbohydr Polym 182:159–171
Monier M, Abdel-Latif DA (2017) Fabrication of Au (III) ion-imprinted polymer based on thiol-modified chitosan. Int J Biol Macromol 105:777–787
Abdulhameed AS, Jawad AH, Mohammad AT (2019) Synthesis of chitosan-ethylene glycol diglycidyl ether/TiO2 nanoparticles for adsorption of reactive orange 16 dye using a response surface methodology approach. Bioresour Technol 293:122071
Akköz Y, Coşkun R, Delibaş A (2019) Preparation and characterization of sulphonated bio-adsorbent from waste hawthorn kernel for dye (MB) removal. J Mol Liq 287:110988
Njoku VO, Islam MA, Asif M, Hameed BH (2014) Preparation of mesoporous activated carbon from coconut frond for the adsorption of carbofuran insecticide. J Anal Appl Pyrol 110:172–180
Lagergren S (1898) Zur theorie der sogenannten adsorption geloster stoffe. Vet Akad Handl 24:1–39
Ho YS, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70:115–124
Muthukumaran C, Sivakumar VM, Thirumarimurugan M (2016) Adsorption isotherms and kinetic studies of crystal violet dye removal from aqueous solution using surfactant modified magnetic nanoadsorbent. J Taiwan Inst Chem Eng 63:354–362
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div 89:31–60
Chen S, Qin C, Wang T, Chen F, Li X, Hou H, Zhou M (2019) Study on the adsorption of dyestuffs with different properties by sludge-rice husk biochar: adsorption capacity, isotherm, kinetic, thermodynamics and mechanism. J Mol Liq 285:62–74
Tanhaei B, Ayati A, Sillanpää M (2019) Magnetic xanthate modified chitosan as an emerging adsorbent for cationic azo dyes removal: Kinetic, thermodynamic and isothermal studies. Int J Biol Macromol 121:1126–1134
Ghaedi AM, Panahimehr M, Nejad ARS, Hosseini SJ, Vafaei A, Baneshi MM (2018) Factorial experimental design for the optimization of highly selective adsorption removal of lead and copper ions using metal organic framework MOF-2 (Cd). J Mol Liq 272:15–26
Salari M, Dehghani MH, Azari A, Motevalli MD, Shabanloo A, Ali I (2019) High performance removal of phenol from aqueous solution by magnetic chitosan based on response surface methodology and genetic algorithm. J Mol Liq 285:146–157
Celekli A, Yavuzatmaca M, Bozkurt H (2009) Kinetic and equilibrium studies on the adsorption of reactive red 120 from aqueous solution on Spirogyra majuscule. Chem Eng J 152:139–145
Çelekli A, Al-Nuaimi AI, Bozkurt H (2019) Adsorption kinetic and isotherms of Reactive Red 120 on moringa oleifera seed as an eco-friendly process. J Mol Struct 1195:168–178
Demarchi CA, Campos M, Rodrigues CA (2013) Adsorption of textile dye Reactive Red 120 by the chitosan–Fe (III)-crosslinked: Batch and fixed-bed studies. J Environ Chem Eng 1:1350–1358
Munagapati VS, Wen JC, Pan CL, Gutha Y, Wen JH (2019) Enhanced adsorption performance of Reactive Red 120 azo dye from aqueous solution using quaternary amine modified orange peel powder. J Mol Liq 285:375–385
Cardoso NF, Lima EC, Royer B, Bach MV, Dotto GL, Pinto LA, Calvete T (2012) Comparison of Spirulina platensis microalgae and commercial activated carbon as adsorbents for the removal of Reactive Red 120 dye from aqueous effluents. J Hazard Mater 241:146–153
Prola LD, Acayanka E, Lima EC, Umpierres CS, Vaghetti JC, Santos WO, Djifon PT (2013) Comparison of Jatropha curcas shells in natural form and treated by non-thermal plasma as biosorbents for removal of Reactive Red 120 textile dye from aqueous solution. Ind Crops Prod 46:328–340
Pereira IC, Carvalho KQ, Passig FH, Ferreira RC, Rizzo-Domingues RCP, Hoppen MI, Perretto F (2018) Thermal and thermal-acid treated sewage sludge for the removal of dye reactive Red 120: Characteristics, kinetics, isotherms, thermodynamics and response surface methodology design. J Environ Chem Eng 6:7233–7246
Hamed MM, Rizk HE, Ahmed IM (2018) Adsorption behavior of zirconium and molybdenum from nitric acid medium using low-cost adsorbent. J Mol Liq 249:361–370
Chen Y, Long W, Xu H (2019) Efficient removal of Acid Red 18 from aqueous solution by in-situ polymerization of polypyrrole-chitosan composites. J Mol Liq 287:110888
Parker HL, Hunt AJ, Budarin VL, Shuttleworth PS, Miller KL, Clark JH (2012) The importance of being porous: polysaccharide-derived mesoporous materials for use in dye adsorption. RSC Adv 2:8992–8997
Singh SK, Das A (2015) The n→ π* interaction: a rapidly emerging non-covalent interaction. Phys Chem Chem Phys 17:9596–9612
Acknowledgements
The authors would like to thank the Ministry of Higher Education (MOHE), Malaysia for supporting this project under Fundamental Research Grant Scheme (FRGS): FRGS/1/2019/STG01/UiTM/02/3, No,. Fail RMC: 600-IRMI/FRGS 5/3 (340/2019).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jawad, A.H., Mubarak, N.S.A. & Abdulhameed, A.S. Hybrid Crosslinked Chitosan-Epichlorohydrin/TiO2 Nanocomposite for Reactive Red 120 Dye Adsorption: Kinetic, Isotherm, Thermodynamic, and Mechanism Study. J Polym Environ 28, 624–637 (2020). https://doi.org/10.1007/s10924-019-01631-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-019-01631-8