Abstract
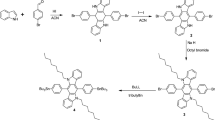
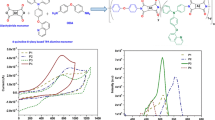
Four donor-acceptor type polymers based on quinoline and biquinoline have been synthesized by Pd catalyzed direct C-H (hetero)arylation reaction. Polymers P1 and P2 are alternate copolymers of thiophene-benzothiadiazole-thiophene (TBTT) unit with quinoline and biquinoline unit, respectively. P3 is a random copolymer containing cyclopentadithiophene (CPDT), benzothiadiazole and quinoline moieties in the backbone whereas P4 contains CPDT unit with randomly distributed benzothiadiazole and biquinoline units. All the polymers show good thermal stability and solubility in common organic solvents. CPDT based polymers P3 and P4 exhibit higher absorbance maxima, higher lying Highest Occupied Molecular Orbital (HOMO) energy levels and smaller band gap as compared to thiophene based polymers P1 and P2 as a result of better electron-donating ability of the former leading to stronger intramolecular charge transfer. Also, quinoline based polymers P1 and P3 show a red-shift in the absorbance maxima compared to biquinoline based polymers P2 and P4, respectively due to non-planar transoid conformation of the two quinoline rings in the biquinoline unit. It is found that the use of N-heterocycle based comonomers allows the tuning of the HOMO level over a remarkably wide range (~0.8 eV). Additionally, the use of quinoline or biquinoline along the conjugated chain leads to deeper lying HOMO levels suggesting good oxidative stability for this class of materials.







Similar content being viewed by others
References
Lu F, Nakanishi T (2015) Alkyl-π engineering in state control toward versatile optoelectronic soft materials. Sci Technol Adv Mater 16(1):14805
Jou JH, Kumar S, Agrawal A, Li TH, Sahoo S (2015) Approaches for fabricating high efficiency organic light emitting diodes. J Mater Chem C 3(13):2974–3002
Kola S, Sinha J, Katz HE (2012) Organic transistors in the new decade: Toward n-channel, printed, and stabilized devices. J Polym Sci B Polym Phys 50(15):1090–1120
Ragoussi M-E, Torres T (2015) New generation solar cells: concepts, trends and perspectives. Chem Commun 51(19):3957–3972
Zhou H, Yang L, Stoneking S, You W (2010) A weak donor-strong acceptor strategy to design ideal polymers for organic solar cells. ACS Appl Mater Interfaces 2:1377–1383
Ajayaghosh A (2003) Donor-acceptor type low band gap polymers: polysquaraines and related systems. Chem Soc Rev 32:181–191
Roncali J (1997) Synthetic principles for bandgap control in linear π-conjugated systems. Chem Rev 97:173–205
Bundgaard E, Krebs FC (2007) Low band gap polymers for organic Photovoltaics. Sol Energy Mater Sol Cells 91:954–985.
Bundgaard E, Krebs FC (2006) Low-band-gap conjugated polymers based on thiophene, benzothiadiazole, and benzobis(thiadiazole). Macromolecules 39(8):2823–2831
Chu TY, Lu J, Beaupre S, Zhang Y, Pouliot J-R, Wakim S, Zhou J, Leclerc M, Li Z, Ding J, Tao Y (2011) Bulk heterojunction solar cells using thieno[3,4-c]pyrrole-4,6-dione and dithieno[3,2-b:2',3'-d]silole copolymer with a power conversion efficiency of 7.3 %. J Am Chem Soc 133:4250–4253
Amb CM, Chen S, Graham KR, Subbiah J, Small CE, So F, Reynolds JR (2011) Dithienogermole as a fused electron donor in bulk heterojunction solar cells. J Am Chem Soc 133:10062–10065
Zhou H, Yang L, Stuart AC, Price SC, Liu S, You W (2011) Development of fluorinated benzothiadiazole as a structural unit for a polymer solar cell of 7 % efficiency. Angew Chem Int Ed Engl 50:2995–2998
Park SH, Roy A, Beaupre S, Cho S, Coates N, Moon JS, Moses D, Leclerc M, Lee K, Heeger AJ (2009) Bulk heterojunction solar cells with internal Quantum efficiency approaching 100 %. Nat Photonics 3:297–303
Price SC, Stuart AC, Yang L, Zhou H, You W (2011) Fluorine substituted conjugated polymer of medium band gap yields 7 % efficiency in polymer-fullerene solar cells. J Am Chem Soc 133:4625–4631
Li Y, Xue L, Li H, Li Z, Xu B, Wen S, Tian W (2009) Energy level and molecular structure engineering of conjugated donor − acceptor copolymers for photovoltaic applications. Macromolecules 42(13):4491–4499
Chen GY, Cheng YH, Chou YJ, Su MS, Chen CM, Wei KH (2011) Crystalline conjugated polymer containing fused 2,5-di(thiophen-2-yl)thieno[2,3-b]thiophene and thieno[3,4-c]pyrrole-4,6-dione units for bulk heterojunction solar cells. Chem Commun 47(17):5064–5066
Jiang JM, Yang PA, Hsieh TH, Wei KH (2011) Crystalline low-band gap polymers comprising thiophene and 2,1,3-benzooxadiazole units for bulk heterojunction solar cells. Macromolecules 44(23):9155–9163
Peet J, Kim JY, Coates NE, Ma WL, Moses D, Heeger AJ, Bazan GC (2007) Efficiency enhancement in low-bandgap polymer solar cells by processing with alkane dithiols. Nat Mater 6:497–500
Biniek L, Chochos CL, Leclerc N, Hadziioannou G, Kallitsis JK, Bechara R, Leveque P, Heiser T (2009) A [3,2-b]thienothiophene-alt-benzothiadiazole copolymer for photovoltaic applications: design, synthesis, material characterization and device performances. J Mater Chem 19(28):4946–4951
Ko S, Mondal R, Risko C, Lee JK, Hong S, McGehee MD, Brédas J-L, Bao Z (2010) Tuning the optoelectronic properties of vinylene-linked donor − acceptor copolymers for organic photovoltaics. Macromolecules 43(16):6685–6698
Liang F, Lu J, Ding J, Movileanu R, Tao Y (2009) Design and synthesis of alternating regioregular oligothiophenes/benzothiadiazole copolymers for organic solar cells. Macromolecules 42:6107–6114
Wu WC, Liu CL, Chen WC (2006) Synthesis and characterization of new fluorene-acceptor alternating and random copolymers for light-emitting applications. Polymer 47(2):527–538
El Shehawy AA, Abdo NI, El Barbary AA, Lee JS (2011) Alternating copolymers based on 2,1,3-benzothiadiazole and hexylthiophene: positioning effect of hexyl chains on the photophysical and electrochemical properties. Eur J Org Chem 25:4841–4852
Botiz I, Schaller RD, Verduzco R, Darling SB (2011) Optoelectronic properties and charge transfer in donor–acceptor all-conjugated diblock copolymers. J Phys Chem C 115(18):9260–9266
Chen MH, Hou J, Hong Z, Yang G, Sista S, Chen LM, Yang Y (2009) Efficient polymer solar cells with thin active layers based on alternating polyfluorene copolymer/fullerene bulk heterojunctions. Adv Mater 21:4238–4242
Shi F, Fang G, Liang F, Wang L, Mu Z, Zhang X, Xie Z, Su Z (2010) Broad absorbing low-bandgap polythiophene derivatives incorporating separate and content-tunable benzothiadiazole and carbazole moieties for polymer solar cells. Eur Polym J 46:1770–1777
Tamilavan V, Song M, Jin S-H, Hyun MH (2011) Synthesis of conjugated polymers with broad absorption bands and photovoltaic properties as bulk heterojuction solar cells. Polymer 52(11):2384–2390
Biniek L, Chochos CL, Hadziioannou G, Leclerc N, Lévêque P, Heiser T (2010) Electronic properties and photovoltaic performances of a series of oligothiophene copolymers incorporating both thieno[3,2-b]thiophene and 2,1,3-benzothiadiazole moieties. Macromol Rapid Commun 31(7):651–656
Padhy H, Huang JH, Sahu D, Patra D, Kekuda D, Chu CW, Lin HC (2010) Synthesis and applications of low-bandgap conjugated polymers containing phenothiazine donor and various benzodiazole acceptors for polymer solar cells. J Polym Sci, Part A: Polym Chem 48:4823–4834
Kanbara T, Saito N, Yamamoto T, Kubota K (1991) Preparation and properties of poly(quinolinediyl)s and poly(isoquinoline-1,4-diyl) with new pi-conjugation systems. Macromolecules 24(21):5883–5885
Saito N, Kanbara T, Nakamura Y, Yamamoto T, Kubota K (1994) Electrochemical and chemical preparation of linear pi-conjugated poly(quinoline-2,6-diyl) using nickel complexes and electrochemical properties of the polymer. Macromolecules 27(3):756–761
Saito N, Yamamoto T (1995) Preparation of new n-type conducting poly(arylene)s by organometallic process and their electrical and optical properties. Synth Met 69(1–3):539–540
Zhang X, Shetty AS, Jenekhe SA (1999) Electroluminescence and photophysical properties of polyquinolines. Macromolecules 32(22):7422–7429
Agrawal AK, Jenekhe SA (1996) Electrochemical properties and electronic structures of conjugated polyquinolines and polyanthrazolines. Chem Mater 8(2):579–589
Zhang X, Shetty AS, Jenekhe SA (1998) Efficient electroluminescence from a new n-type conjugated polyquinoline. Acta Polym 49(1):52–55
Kim JL, Kim JK, Cho HN, Kim DY, Kim CY, Hong SI (2000) New polyquinoline copolymers: synthesis, optical, luminescent, and hole-blocking/electron-transporting properties. Macromolecules 33(16):5880–5885
Tomar M, Lucas NT, Kim H, Laquai F, Müllen K, Jacob J (2012) Facile synthesis of 5,8-linked quinoline-based copolymers. Polym Int 61(8):1318–1325
Tomar M, Lucas NT, Gardiner MG, Muellen K, Jacob J (2012) Facile synthesis and coupling of functionalized isomeric biquinolines. Tetrahedron Lett 53(3):285–288
Drozdov FV, Myshkovskaya EN, Susarova DK, Troshin PA, Fominykh OD, Balakina MY, Bakirov AV, Shcherbina MA, Choi J, Tondelier D (2013) Novel cyclopentadithiophene based D–A copolymers for organic photovoltaic cell applications. Macromol Chem Phys 214(19):2144–2156
Pilgram K, Zupan M, Skiles R (1970) Bromination of 2,1,3-benzothiadiazoles. J Heterocycl Chem 7(3):629–633
Kim J, Yun MH, Anant P, Cho S, Jacob J, Kim JY, Yang C (2011) Copolymers comprising 2,7-carbazole and bis-benzothiadiazole units for bulk-heterojunction solar cells. Chem Eur J 17(51):14681–14688
Acknowledgments
The authors acknowledge the Department of Science and Technology, India (SB/S1/OC-12/2013) and Max Planck Society, Germany for generous financial support. M.T. acknowledges a research fellowship from the Indian Institute of Technology Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tomar, M., Ashar, A.Z., Narayan, K.S. et al. Tuning the HOMO energy levels in quinoline and biquinoline based donor-acceptor polymers. J Polym Res 23, 50 (2016). https://doi.org/10.1007/s10965-016-0945-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-016-0945-1