Abstract
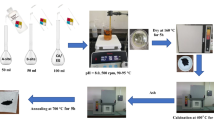
We first introduce the latest experimental results, i.e., production of the fine nanostructured and near fully dense transparent Y3Al5O12 (YAG) bulks at high pressure and modest temperature (2.0–5.0 GPa and 300–500 °C). And then, we employ the first-principles plane wave pseudopotential density functional theory method to calculate the equilibrium lattice parameters and the thermodynamic properties of YAG. The obtained lattice parameters are consistent with the experimental data and the available theoretical data of others. Through the quasi-harmonic Debye model, the dependences of the normalized primitive volume V/V 0 on pressure P, the Debye temperature \( \Uptheta_{\rm{D}} \), and the heat capacity C V on pressure P and temperature T, as well as the variation of the thermal expansion α with temperature and pressure are obtained successfully.






Similar content being viewed by others
References
Yoder HS, Keith ML. Complete substitution of aluminium for silicon: the system 3MnO·Al2O3·3SiO2–3Y2O3·5Al2O3. Am Mineralogist. 1951;36:519–33.
Rabinovitch Y, Tétard D, Faucher MD, Pham-Thi M. Transparent polycrystalline neodymium doped YAG: synthesis parameters, laser efficiency. Opt Mater. 2003;24:345–51.
Saito N, Matsuda SI, Ikegami T. Fabrication of transparent yttria ceramics at low temperature using carbonate derived powder. J Am Ceram Soc. 1998;81:2023–8.
Zych E, Hreniak D, Stręk W, Kępiński L, Domagała K. Sintering properties of urea-derived Lu2O3-based phosphors. J Alloys Compd. 2002;341:391–4.
Ikesue A, Furusato I, Kamata K. Fabrication of polycrystalline, transparent YAG ceramics by a solid-state reaction method. J Am Ceram Soc. 1995;78(1):225–8.
Ikesue A, Kinoshita T, Kamata K, Yoshida K. Fabrication and optical properties of high-performance polycrystalline Nd:YAG ceramics for solid-state lasers. J Am Ceram Soc. 1995;78:1033–40.
Durrani SK, Saeed K, Qureshi AH, Hmad MA, Arif M, Hussain N, Mohammad T. Growth of Nd-doped YAG powder by sol spray process. J Therm Anal Calorim. 2010;104:645–51.
Carvalho JF, De Vicente FS, Marcellin N, Odier P, Hernandes AC, Ibanez A. Synthesis of YAP phase by a polymeric method and phase progression mechanisms. J Therm Anal Calorim. 2009;96:891–6.
Fedyk R, Hreniak D, Łojkowski W, Strek W, Matysiak H, Grzanka E, Gierlotka S, Mazur P. Method of preparation and structural properties of transparent YAG nanoceramics. Opt Mater. 2007;29:1252–7.
Pazik R, Głuchowski P, Hreniak D, Strek W, Roś M, Fedyk R, Łojkowski W. Fabrication and luminescence studies of Ce:Y3Al5O12 transparent nanoceramic. Opt Mater. 2008;30:714–8.
Hreniak D, Strek W, Głuchowski P, Fedyk R, Łojkowski W. The concentration dependence of luminescence of Nd:Y3Al5O12 nanoceramics. J Alloys Compd. 2008;451:549–52.
Lukowiak A, Wiglusz RJ, Maczka M, Gluchowski P, Strek W. IR and Raman spectroscopy study of YAG nanoceramics. Chem Phys Lett. 2010;494:279–83.
Hreniak D, Fedyk R, Bednarkiewicz A, Stre W, Łojkowski W. Luminescence properties of Nd:YAG nanoceramics prepared by low temperature high pressure sintering method. Opt Mater. 2007;29:1244–51.
Hreniak D, Gierlotka S, Łojkowski W, Strek W, Mazur P, Fedyk R. High-pressure induced structural decomposition of RE-doped YAG nanoceramics. Diffus Defect Data B. 2005;106:17–22.
Vovk EA, Deineka TG, Doroshenko AG, Tkachenko VF, Tolmachev AV, Yakovetskii RP, Petrusha IA, Tkach VN, Turkevich VZ, Danilenko NI. Production of the Y3Al5O12 transparent nanostructured ceramics. J Superhard Mater. 2009;31:252–9.
Yavetskiy RP, Vovk EA, Doroshenko AG, Danylenko MI, Lopin AV, Petrusha IA, Tkachenko VF, Tolmachev AV, Turkevich VZ. Y3Al5O12 translucent nanostructured ceramics-obtaining and optical properties. Ceram Int. 2011;37:2477.
Liu K, He DW, Wang HM, Lu TC, Li F, Zhou XL. High-pressure sintering mechanism of yttrium aluminum garnet (Y3Al5O12) transparent nanoceramics. Scripta Mater. 2012;66:319–22.
Xu YN, Ching WY. Electronic structure of yttrium aluminium garnet. Phys Rev B. 1999;59:10530–5.
Muñoz-García AB, Anglada E, Seijo L. First-principles study of the structure and the electronic structure of yttrium aluminum garnet Y3Al5O12. Int J Quantum Chem. 2009;109:1991–8.
Tong SH, Lu TC, Guo W. Synthesis of YAG powder by alcohol–water co-precipitation method. Mater Lett. 2007;61:4287–9.
Scherrer P. Estimation of the size and internal structure of colloidal particles by means of röntgen. Nachr Ges Wiss Götingen, Math-Pys Kl. 1981;2:96–100.
Chen C, He DW, Kou ZL, Peng F, Yao L, Yu RC, Bi Y. B6O-based composite to rival polycrystalline cubic boron nitride. Adv Mater. 2007;19:4288–91.
Li JG, Ikegami T, Lee JH, Mori T. Low-temperature fabrication of transparent yttrium aluminum garnet (YAG) ceramics without additives. J Am Ceram Soc. 2000;83:961–3.
Segall MD, Linda PLD, Probert MJ, Pickard CJ, Hasnip PJ, Clark SJ, Payne MC. First-principles simulation: ideas, illustrations and the CASTEP code. J Phys. 2002;14:2717–43.
Vanderbilt D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys Rev B. 1990;4:7892–5.
Perdew JP, Wang Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys Rev B. 1992;45:13244–9.
Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys Rev Lett. 1996;77:3865–8.
Ceperley DM, Alder BJ. Ground state of the electron gas by a stochastic method. Phys Rev Lett. 1980;45:566–9.
Monkhorst J, Pack JD. Special points for brillouin-zone integrations. Phys Rev B. 1976;13:5188–92.
Lim AR. Thermodynamic properties and phase transitions of Tutton salt (NH4)2Co(SO4)2·6H2O crystals. J Therm Anal Calorim. 2011;. doi:10.1007/s10973-011-1849-2.
Atanasova L, Baikusheva-Dimitrova G. Heat capacity and thermodynamic properties of tellurites Yb2(TeO3)3, Dy2(TeO3)3 and Er2(TeO3)3. J Therm Anal Calorim. 2011;. doi:10.1007/s10973-011-1325-z.
Xue BD, Yang Q, Chen SP, Gao SL. Synthesis, crystal structure, and thermodynamics of a high-nitrogen copper complex with N,N-bis-(1(2)H-tetrazol-5-yl) amine. J Therm Anal Calorim. 2010;101:997–1002.
Knyazev A, Maczka M, Kuznetsova N, Hanuza J, Markin A. Thermodynamic properties of rubidium niobium tungsten oxide. J Therm Anal Calorim. 2009;98:843–8.
Li XY, Wu YQ, Gu DH, Gan FX. Synthesis, spectral and thermal properties of some transition metal(II) complexes with a novel ligand derived from thiobarbituric acid. J Therm Anal Calorim. 2009;98:387–94.
Blanco MA, Francisco E, Luana V. GIBBS: isothermal-isobaric thermodynamics of solids from energy curves using a quasi-harmonic Debye model. Comput Phys Commun. 2004;158:57–72.
Guo HZ, Chen XR, Cai LC, Zhu J, Gao J. Structural and thermodynamic properties of MgB2 from first-principles calculations. Solid State Commun. 2005;134:787–90.
Chen XR, Wang HY, Cheng Y, Hao YJ. First-principles calculations for structure and equation of state of MgB2 at high pressure. Phys B. 2005;307:281–6.
Guo HZ, Chen XR, Cai LC, Zhu J, Gao J. First-principles calculations of elastic constants of superconducting MgB2. Chin Phys Lett. 2005;. doi:10.1088/0256-307X/22/7/056.
Zhou XL, Liu K, Chen XR, Zhu J. Structural and thermodynamic properties of AlB2 compound. Chin Phys. 2006;15:3014–8.
Liu K, He DW, Zhou XL, Chen HH. First-principles study of structural and thermodynamic properties of osmium. Phys B. 2011;406:3065–9.
Liu K, Zhou XL, Chen HH, Lu LY. Phase transition and thermodynamic properties of TiN under pressure via first-principles calculations. J Therm Anal Calorim. 2011;. doi:10.1007/s10973-011-1927-5.
Lu LY, Chen XR, Yu BR, Gou QQ. First-principles calculations for transition phase and thermodynamic properties of GaAs. Chin Phys. 2006;. doi:10.1088/1009-1963/15/4/022.
Hu CE, Zeng ZY, Cheng Y, Chen XR, Cai LC. First-principles calculations for electronic, optical and thermodynamic properties of ZnS. Chin Phys B. 2008;. doi:10.1088/1674-1056/17/10/053.
Lu LY, Chen XR, Chen Y, Zhou JZ. Transition phase and thermodynamic properties of GaN via first-principles calculations. Solid State Commun. 2005;136:152–6.
Blanco MA, Pendás AM, Francisco E, Recio JM, Franco R. Thermodynamical properties of solids from microscopic theory: applications to MgF2 and Al2O3. J Mol Struct (Theochem). 1996;368:245–55.
Flórez M, Recio JM, Francisco E, Blanco MA, Pendás AM. First-principles study of the rock salt-cesium chloride relative phase stability in alkali halides. Phys Rev B. 2002;66:144112/1–8.
Francisco E, Sanjurjo MA. Atomistic simulation of SrF2 polymorphs. Phys Rev B. 2001;63:094107/1–9.
Yu R, Zhang XF. Platinum nitride with fluorite structure. Appl Phys Lett. 2005;86:121913–5.
Stampfl C, Mannstadt W, Asahi R, Freeman AJ. Electronic structure and physical properties of early transition metal mononitrides: density-functional theory LDA, GGA, and screened-exchange LDA FLAPW calculations. Phys Rev B. 2001;63:155106–16.
Poirier JP, Tarantol A. A logarithmic equation of state. Phys Earth Planet Int. 1998;109:1–8.
Euler F, Bruce JA. Oxygen coordinates of compounds with garnet structure. Acta Crystallogr. 1965;19:971–8.
Hofmeiser AM, Campbell KR. Infrared spectroscopy of yttrium aluminum, yttrium gallium, and yttrium iron garnets. J Appl Phys. 1992;72:638–46.
Stoddart PR, Ngoepe PE, Mjwara PM, Comins JD, Saunders GA. High-temperature elastic constants of yttrium aluminum garnet. J Appl Phys. 1993;73:7298–301.
Alton WJ, Barow AJ. Elastic constants of single-crystal YIG. J Appl Phys. 1967;32:1172–3.
Yogurtcu YK, Miller AJ, Saunders GA. Elastic behavior of YAG under pressure. J Phys C. 1980;13:6585–97.
Aggarwal RL, Ripin DJ, Ochoa JR, Fan TY. Measurement of thermo-optic properties of Y3Al5O12, Lu3Al5O12, YAIO3, LiYF4, LiLuF4, BaY2F8, KGd(WO4)2, and KY(WO4)2 laser crystals in the 80–300 K temperature range. J Appl Phys. 2005;98:103514–27.
Acknowledgements
This study was supported by the China 973 Program (Grant No. 2011CB808200), the National Natural Science Foundation of China (Grant No. 11027405), the National Natural Science Foundation of China—NSAF (Grant No. 10976018) and Scientific Research Fund of Sichuan Provincial Education Department (Grant No. 09ZDL01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, K., He, DW., Zhou, XL. et al. Method of preparation and thermodynamic properties of transparent Y3Al5O12 nanoceramics. J Therm Anal Calorim 111, 289–294 (2013). https://doi.org/10.1007/s10973-012-2307-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2307-5