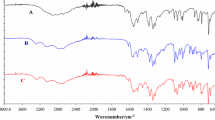
Abstract
In this paper, we present the spectroscopic description (by the means of UATR-FTIR spectroscopy) for four naturally occurring bile acids, namely lithocholic acid (LC), taurocholic acid sodium salt hydrate (TC), taurodeoxycholic acid sodium salt hydrate (TDC) and chenodeoxycholic acid (CDC), as well as their thermal behaviour in dynamic oxidative atmosphere, by TG/DTG/HF means. It was shown that all samples have a good thermal stability, favourized by the presence of cholan-24-oic structural moiety in all samples, as well as the solid-state structure (intermolecular H-bondings for the carboxylic acids CDC and LC and saline structures for TDC and TC, respectively). These data are simple, but of great interest in identifying the presence of these compounds by fast, reproducible and precise instrumental techniques in complex mixtures, such as biliary stones.



Similar content being viewed by others
References
Burkard I, Von Eckardstein A, Rentsch KM. Differentiated quantification of human bile acids in serum by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B. 2005;826:147–59.
Zígolo MA, García Liñares G, Baldessari A. New cholic acid derivatives: biocatalytic synthesis and molecular docking study. Steroids. 2016;107:10–9.
Borzellino G, Cordiano C. Biliary lithiasis. Milan: Springer; 2008.
Chuang SC, Hsi E, Lee KT. Genetics of gallstone disease. Adv Clin Chem. 2013;60:143–85.
Gallagher TK, Parks RW. Gallstones Surg. 2014;32(12):635–42.
Chowdhury AH, Lobo DN. Gallstones Surg. 2011;29(12):610–7.
Paracha PI, Asif Y, Vriesekoop F, Ullah S, Abbas M, Paracha SI, Khan T. Risk factors associated with gallstone disease in women. E-SPEN J. 2012;7(3):e129–34.
Cetta F, Lombardo F, Giubbolini M, Baldi C, Cariati A. Classification of gallstones and epidemiologic studies. Dig Dis Sci. 1995;40(10):2189–91.
Fu P, Zhang S, Dai K, Zheng KZC. Gallstone classified based on sectional structure and chemical composition. Chin J Surg. 1984;22(5):258–60.
Castro-Torres IG, de Jesús Cárdenas-Vázquez R, Velázquez-González C, Ventura-Martínez R, De la O-Arciniega M, Naranjo-Rodríguez EB, Martínez-Vázquez M. Future therapeutic targets for the treatment and prevention of cholesterol gallstones. Eur J Pharmacol. 2015;765:366–74.
Buda V, Andor M, Ledeti A, Ledeti I, Vlase G, Vlase T, Cristescu C, Voicu M, Suciu L, Tomescu C. Comparative solid-state stability of perindopril active substance vs. pharmaceutical formulation. Int J Mol Sci. 2017;18(1):164–79.
Fulias A, Vlase G, Vlase T, Onetiu D, Doca N, Ledeti I. Thermal degradation of B-group vitamins: B-1, B-2 and B-6. J Therm Anal Calorim. 2014;118(2):1033–84.
Ledeti I, Vlase G, Vlase T, Doca N, Bercean V, Fulias A. Thermal decomposition, kinetic study and evolved gas analysis of 1,3,5-triazine-2,4,6-triamine. J Therm Anal Calorim. 2014;118(2):1057–63.
Ledeti I, Vlase G, Vlase T, Doca N, Fulias A, Suta LM. Comparative thermal stability of two similar-structure hypolipidemic agents Simvastatin and Lovastatin-kinetic study. J Therm Anal Calorim. 2016;125(2):769–75.
Ledeti A, Vlase G, Vlase T, Bercean V, Murariu MS, Ledeti I, Suta LM. Solid-state preformulation studies of amiodarone hydrochloride. J Therm Anal Calorim. 2016;126(1):181–7.
Lamcharfi E, Cohen-Solal C, Marquet M, Lutton C, Dupre J, Meyer C. Determinations of molecular associations of some hydrophobic and hydrophilic bile acids by infrared and Raman spectroscopy. J Euro Biophys. 1997;54(4):285–91.
Rudzki A, Ossowska-Chruściel MD, Ordon M, Zając W, Chruściel J. Thermal analysis and simulation model of natural lithocholic acid. J Therm Anal Calorim. 2015;122(1):55–64.
Levaray N, Zhu XX. Polyurethanes made from bile acids. Chin J Polym Sci. 2016;34(5):616–22.
Yang L, Xu Y, Su Y, Wu J, Zhao K, Chen J, Wang M. FT-IR spectroscopic study on the variations of molecular structures of some carboxyl acids induced by free electron laser. Spectrochim Acta Part A Mol Biomol Spectrosc. 2005;62(4–5):1209–15.
Budavari S, editor. The Merck index: an encyclopedia of chemicals, drugs, and biologicals. Whitehouse Station: Merck and Co., Inc.; 1996. p. 946.
Acknowledgements
This work was supported by a grant financed by the University of Medicine and Pharmacy “Victor Babes” Timisoara (Grant PIII-C3-PCFI-2016/2017, acronym STONES).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ledeti, I., Pusztai, A.M., Murariu, M. et al. Comparative instrumental investigations of some bile acids. J Therm Anal Calorim 134, 1345–1350 (2018). https://doi.org/10.1007/s10973-018-7163-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7163-5