Abstract
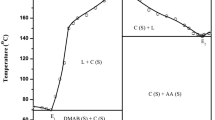
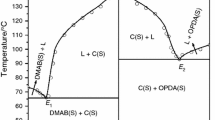
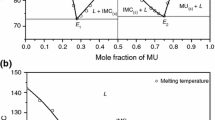
The phase diagram of anthranilic acid (AA)–m-nitro benzoic acid (NBA) system, determined by the thaw–melt method, shows the formation of a 2:1 (AA:NBA) inter-molecular compound (IMC) and two eutectics E1 and E2 containing 0.084 and 0.917 mol fractions of AA, respectively, with one eutectic on either side of the IMC. The heat of mixing, entropy of fusion, roughness parameter, interfacial energy, and the excess thermodynamic functions were calculated from the enthalpy of fusion data, obtained by the DSC method. While the spectroscopic investigations (IR and NMR) and optical studies suggest the presence of hydrogen bonding between the components, the powder X-ray diffraction spectra of eutectics and the IMC suggest that the eutectic E1 is a mechanical mixture of the IMC and NBA and the eutectic E2 is a mechanical mixture of the IMC and AA. A single crystal of cocrystal of AA and NBA was grown using slow evaporation technique at room temperature from its saturated solution in methanol solvent. Single-crystal analysis of the grown cocrystal shows the mode of the intermolecular hydrogen bonding in the molecule and the triclinic crystal structure with P − 1 space group.






Similar content being viewed by others
References
Sangster J. Phase diagrams and thermodynamic properties of binary organic systems based on 1,2-,1,3-,1,4-diaminobenzene or benzidine. J Phys Chem Ref Data. 1994;23(2):295–338.
Rai RN, Reddi RSB, Rai US. Developments and future directions of phase diagram, physicochemical and optical studies of binary organic complexes. Prog Cryst Growth Charact Mater. 2013;59(2):73–111.
Okumuş M. Thermal characterization of binary mixture of some supramolecular liquid crystals. J Therm Anal Calorim. 2015;120(3):1603–8.
Rai US, Singh M. An overview of the progress in solidification of binary monotectics. J Mater Chem Eng. 2013;1(2):75–84.
Singh NB, Agrawal T, Gupta P, Das SS. Solidification behavior of the benzamide + O-chlorobenzoic acid eutectic system. J Chem Eng Data. 2009;54(5):1529–36.
Chem YP, Tang M, Cuo TC. Solid–liquid equilibria for binary mixtures of N-phenylacetamide with 4-aminoacetophenone, 3-hydroxyacetophenone and 4-hydroxyacetophenone. Fluid Phase Equilib. 2005;232(2):182–8.
Rai RN, Ramasamy P, Lan CW. Synthesis and crystal growth of binary organic NLO material UNBA. J Cryst Growth. 2002;235(1):499–504.
Horiuchi S, Tokura Y. Organic ferroelectrics. Nat Mater. 2008;7(5):357–66.
Horiuchi S, Kumari R, Tokura Y. A supramolecular ferroelectric realized by collective proton transfer. Angew Chem Int Ed. 2007;46(19):3497–501.
Abbott AP, Taib KE, Frisch G, Ryder KS, Weston D. The electrodeposition of silver composites using deep eutectic solvents. Phys Chem Chem Phys. 2012;14(7):2443–9.
Sechiguchi K, Obi N. Studies on absorption of eutectic mixture. I. A comparison of the behavior of eutectic mixture of sulfathiazole and that of ordinary sulfathiazole in man. Chem Pharm Bull. 1961;9(11):866–72.
Cherukuvada S, Nangia A. Eutectics as improved pharmaceutical materials: design, properties and characterization. Chem Commun. 2014;50(8):906–23.
Springwl G, Norberg B, Robeyns K, Wouters J, Leyssess T. Advances in pharmaceutical co-crystal screening: effective co-crystal screening through structural resemblance. Cryst Growth Des. 2012;12(1):475–84.
Desiraju GR. Supramolecular synthons in crystal engineering—a new organic synthesis. Angew Chem Int Ed Engl. 1995;34:2311–27.
Fan E, Vincent C, Geib SJ, Hamilton AD. Molecular recognition in the solid state: hydrogen-bonding control of molecular aggregation. Chem Mater. 1994;6:1113–7.
Dean JA. Lange’s handbook of chemistry. New York: McGraw-Hill; 1985.
Singh M, Rai RN, Rai US. Synthesis, crystal growth and physicochemical studies on a novel organic inter-molecular compound; 3,5-dinitrobenzoic acid and salicylamide system. J Cryst Growth. 2015;419:114–22.
Reddi RSB, Kant S, Rai US, Rai RN. Crystallization, thermal, phase diagram and microstructural studies of organic analog of metal–nonmetal monotectic alloy: 4-bromochlorobenzene–succinonitrile. J Cryst Growth. 2009;312:95–9.
Sheldirck GM. Shelex-97, program for crystal structure refinement from diffraction data. Gottingen: University of Gottingen; 1997.
Rai US, Singh M, Rai RN. Solid state synthesis, structural, physicochemical and optical properties of an inter-molecular compound: 2-hydroxy-1,2-diphenylethanone-4-nitro-ophenylenediamine system. J Solid State Chem. 2017;253:63–72.
Rai RN, Verma KBR. Phase diagram and dielectric studies of binary organic materials. Mater Lett. 2000;44:284–93.
Singh M, Rai US, Rai RN. Some physicochemical and thermal studies on organic analog of a nonmetal-nonmetal monotectic alloy; 2-cyanoacetamide–4-chloronitrobenzene system. Am J Anal Chem. 2011;2:953–61.
Reddi RSB, Ganesamoorthy S, Gupta PK, Rai RN. Phase equilibria, crystallization, thermal and microstructural studies on organic monotectic analog of nonmetal–nonmetal system; urea–4-bromo-2-nitroaniline. Fluid Phase Equilib. 2012;313:121–6.
Rai US, Singh M, Rai RN. Some physicochemical studies on organic eutectics and intermolecular compounds. J Therm Anal Calorim. 2017. https://doi.org/10.1007/s10973-017-6429-7.
Reddi RSB, Kumar Satuluri VSA, Rai US, Rai RN. Thermal, physicochemical and microstructural studies of binary organic eutectic systems. J Therm Anal Calorim. 2012;107:377–85.
Reddi RSB, Kumar Satuluri VSA, Rai RN. Solid–liquid equilibrium, thermal and physicochemical studies of organic eutectics. J Therm Anal Calorim. 2012;107:183–8.
Rai US, Singh M, Rai RN. Green synthesis, characterization and some physico-chemical studies on a novel intermolecular compound; 4-nitro-ophenylenediamine-N,N-dimethylaminobenzaldehyde system. J Mol Struct. 2017;1144:41–8.
Kalsi PS. Spectroscopy of organic compounds. 6th ed. India: New Age Publication; 2005.
Naumov P, Ohashi Y. Packing-dependent photochromism: the case of photoinduced intramolecular proton transfer in 6-(2′,4′-dinitrobenzyl)-2,2′-bipyridine. Acta Cryst. 2004;B60:343–9.
Acknowledgements
Authors thank the University Grant Commission (UGC), India, and BHU for financial support to MS.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rai, U.S., Singh, M. & Rai, R.N. Green synthesis, crystal growth, and some physicochemical studies on an inter-molecular compound of anthranilic acid and m-nitro-benzoic acid system. J Therm Anal Calorim 134, 1001–1009 (2018). https://doi.org/10.1007/s10973-018-7344-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7344-2