Abstract
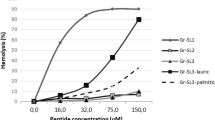
Tuberculosis is one of the leading causes of death, with an annual mortality rate of 2 million. The present treatment regimen for Mycobacterium species is strenuous, extending up to 12 months. Even then, rise of antibiotic resistance has limited the prognosis, with increased instances of multidrug resistant (MDR–TB) and extremely drug resistant (XDR–TB) cases reported. Peptide based antibiotics can be an effective solution due to their low toxicity, biocompatibility and predictable metabolism, but has not been employed due to their short plasma half life. In this brief communication, we demonstrate the bactericidal potency of cationic amphipathic peptides as an effective bactericidal agent against Mycobacterium smegmatis. Potency of stereo-engineered LDLD or DLDL peptides have been retained their potency, while their poly L variants rapidly lost their activity, when the experiment was repeated in human serum. To establish this as a design strategy, we further verified the results by repeating the experiment in a gram negative bacteria E. coli. One of the designed peptides were showing a minimum inhibitory concentration (MIC) value as low as 3.13 µM, suggesting the possibility of future development as a therapeutic peptide.



Similar content being viewed by others
References
Albada HB, Prochnow P, Bobersky S, Bandow JE, Metzler-Nolte N (2014) Highly active antibacterial ferrocenoylated or ruthenocenoylated Arg-Trp peptides can be discovered by an l-to-d substitution scan. Chem Sci 5:4453–4459
Anderson L (2005) Candidate-based proteomics in the search for biomarkers of cardiovascular disease. J Physiol 563:23–60
Arakha M, Borah SM, Saleem M, Jha AN, Jha S (2016) Interfacial assembly at silver nanoparticle enhances the antibacterial efficacy of nisin. Free Radic Biol Med 101:434–445
Bals R, Goldman MJ, Wilson JM (1998) Mouse β-defensin 1 is a salt-sensitive antimicrobial peptide present in epithelia of the lung and urogenital tract. Infect Immun 66:1225–1232
Beg S, Gaur P, Mishra S (2017) New drugs and vaccines for tuberculosis. Recent Pat Anti-Infect Drug Discov. https://doi.org/10.2174/1574891X12666171006105921
Billmeyer FW (1971) Textbook of polymer science. Wiley, New York
Brannon JR, Thomassin J-L, Gruenheid S, Le Moual H (2015) Antimicrobial Peptide conformation as a structural determinant of omptin protease specificity. J Bacteriol 197:3583–3591
Burkhart BM, Gassman RM, Langs DA, Pangborn WA, Duax WL, Pletnev V (1999) Gramicidin D conformation, dynamics and membrane ion transport. Biopolymers 51:129–144
Carranza-Rosales P et al (2017) Modeling tuberculosis pathogenesis through ex vivo lung tissue infection. Tuberculosis 107:126–132
Chaudhary N, Nagaraj R (2011) Impact on the replacement of Phe by Trp in a short fragment of Aβ amyloid peptide on the formation of fibrils. J Pep Sci 17:115–123
Chung GA, Aktar Z, Jackson S, Duncan K (1995) High-throughput screen for detecting antimycobacterial agents. Antimicrob Agents Chemother 39:2235–2238
Di L (2015) Strategic approaches to optimizing peptide ADME properties. AAPS J 17:134–143
Dosler S, Karaaslan E, Alev Gerceker A (2016) Antibacterial and anti-biofilm activities of melittin and colistin, alone and in combination with antibiotics against Gram-negative bacteria. J Chemother 28:95–103
Durani S (2008) Protein design with l- and d-α-amino acid structures as the alphabet. Acc Chem Res 41:1301–1308
Estrella J et al (2011) A novel in vitro human macrophage model to study the persistence of Mycobacterium tuberculosis using vitamin D3 and retinoic acid activated THP-1 macrophages. Front Microbiol 2:1–16
Falzon D, Schünemann HJ, Harausz E, González-Angulo L, Lienhardt C, Jaramillo E, Weyer K (2017) World Health Organization treatment guidelines for drug-resistant tuberculosis, 2016 update. Eur Respir J 49:1602308
Fjell CD, Hiss JA, Hancock REW, Schneider G (2012) Designing antimicrobial peptides: form follows function. Nat Rev Drug Discov 11:37–51
Fofana MO, Shrestha S, Knight GM, Cohen T, White RG, Cobelens F, Dowdy DW (2017) A multistrain mathematical model to investigate the role of pyrazinamide in the emergence of extensively drug-resistant tuberculosis. Antimicrob Agents Chemother 61:e00498–e00416
Fosgerau K, Hoffmann T (2015) Peptide therapeutics: current status and future directions. Drug Discov Today 20:122–128
Ghadiri MR, Granja JR, Milligan RA, McRee DE, Khazanovich N (1993) Self-assembling organic nanotubes based on a cyclic peptide architecture. Nature 366:324–327
Greene CH, Power MH (1931) The distribution of electrolytes between serum and the in vivo dialysate. J Biol Chem 91:183–202
Gutsmann T (2016) Interaction between antimicrobial peptides and mycobacteria. Biochim Biophys Acta Biomembr 1858:1034–1043
Hazam PK, Jerath G, Chaudhary N, Ramakrishnan V (2017a) Peptido-mimetic approach in the design of syndiotactic antimicrobial peptides. Int J Pept Res Ther. https://doi.org/10.1007/s10989-017-9615-3
Hazam PK, Jerath G, Kumar A, Chaudhary N, Ramakrishnan V (2017b) Effect of tacticity-derived topological constraints in bactericidal peptides. Biochim Biophys Acta Biomembr 1859:1388–1395
Henry RR, Rosenstock J, Logan D, Alessi T, Luskey K, Baron MA (2014) Continuous subcutaneous delivery of exenatide via ITCA 650 leads to sustained glycemic control and weight loss for 48 weeks in metformin-treated subjects with type 2 diabetes. J Diabetes Complicat 28:393–398
Horne WS, Wiethoff CM, Cui C, Wilcoxen KM, Amorin M, Ghadiri MR, Nemerow GR (2005) Antiviral cyclic d,l-alpha-peptides: targeting a general biochemical pathway in virus infections. Bioorg Med Chem 13:5145–5153
Jaskiewicz M, Orlowska M, Olizarowicz G, Migon D, Grzywacz D, Kamysz W (2016) Rapid screening of antimicrobial synthetic peptides. Int J Pept Res Ther 22:155–161
Jenssen H, Aspmo SI (2008) Serum stability of peptides. In: Otvos L (ed) Peptide-based drug design. Humana Press, Totowa, pp 177–186
Jenssen H, Hamill P, Hancock REW (2006) Peptide antimicrobial agents. Clin Microbiol Rev 19:491–511
Jiang Z, Higgins MP, Whitehurst J, Kisich KO, Voskuil MI, Hodges RS (2011) Anti-tuberculosis activity of alpha-helical antimicrobial peptides: de novo designed l- and d-enantiomers versus L- and D-LL-37. Protein Pept Lett 18:241–252
Joo H-S, Fu C-I, Otto M (2016) Bacterial strategies of resistance to antimicrobial peptides. Philos Trans R Soc B 371:20150292
Kaur KJ, Sarkar P, Nagpal S, Khan T, Salunke DM (2008) Structure–function analyses involving palindromic analogs of tritrypticin suggest autonomy of anti-endotoxin and antibacterial activities. Protein Sci 17:545–554
Khusro A, Aarti C, Agastian P (2016) Anti-tubercular peptides: a quest of future therapeutic weapon to combat tuberculosis. Asian Pac J Trop Med 9(11):1023–1034
Koh JJ, Lin S, Aung TT, Lim F, Zou H, Bai Y, Li J, Lin H, Pang LM, Koh WL, Salleh SM, Lakshminarayanan R, Zhou L, Qiu S, Pervushin K, Verma C, Tan DT, Cao D, Liu S, Beuerman RW (2015) Amino acid modified xanthone derivatives: novel, highly promising membrane-active antimicrobials for multidrug-resistant Gram-positive bacterial infections. J Med Chem 58:739–752
Kohlmorgen B, Elias J, Schoen C (2017) Improved performance of the artus Mycobacterium tuberculosis RG PCR kit low incidence setting: a retrospective monocentric study. Sci Rep 7:14127
Kooi C, Sokol PA (2009) Burkholderia cenocepacia zinc metalloproteases influence resistance to antimicrobial peptides. Microbiology 155:2818–2825
Kumar A, Ramakrishnan V (2010) Creating novel protein scripts beyond natural alphabets. Syst Synth Biol 4:247–256
Kumar A, Ranbhor R, Patel K, Ramakrishnan V, Durani S (2017) Automated protein design: landmarks and operational principles. Prog Biophys Mol Biol 125:24–35
Lan Y, Lam JT, Siu GKH, Yam WC, Mason AJ, Lam JKW (2014) Cationic amphipathic d-enantiomeric antimicrobial peptides with in vitro and ex vivo activity against drug-resistant Mycobacterium tuberculosis. Tuberculosis 94:678–689
Li S, Tang B, He H (2016) An imbalanced learning based MDR-TB early warning system. J Med Syst 40:164
Lienhardt C et al (2017) Target regimen profiles for treatment of tuberculosis: a WHO document. Eur Respir J 49:1602352
Mishra B, Wang G (2012) Ab initio design of potent anti-MRSA peptides based on database filtering technology. J Am Chem Soc 134:12426–12429
Mogi T, Murase Y, Mori M, Shiomi K, Omura S, Paranagama MP, Kita K (2009) Polymyxin B identified as an inhibitor of alternative NADH dehydrogenase and malate: quinone oxidoreductase from the Gram-positive bacterium Mycobacterium smegmatis. J Biochem 146:491–499
Molchanova N, Hansen P, Franzyk H (2017) Advances in development of antimicrobial peptidomimetics as potential drugs. Molecules 22:1430
Moncla BJ, Pryke K, Rohan LC, Graebing PW (2011) Degradation of naturally occurring and engineered antimicrobial peptides by proteases. Adv Biosci Biotechnol 2:404–408
Nguyen LT, Chau JK, Perry NA, de Boer L, Zaat SAJ, Vogel HJ (2010) Serum stabilities of short tryptophan- and arginine-rich antimicrobial peptide analogs. PLoS ONE 5:e12684
Nizet V (2006) Antimicrobial peptide resistance mechanisms of human bacterial pathogens. Curr Issues Mol Biol 8:11–26
Olli S, Rangaraj N, Nagaraj R (2013) Effect of selectively introducing arginine and d-amino acids on the antimicrobial activity and salt sensitivity in analogs of human beta-defensins. PLoS ONE 8:e77031
Oren Z, Shai Y (1997) Selective lysis of bacteria but not mammalian cells by diastereomers of melittin: structure—function study. Biochemistry 36:1826–1835
Park IY, Cho JH, Kim KS, Kim Y-B, Kim MS, Kim SC (2004) Helix stability confers salt resistance upon helical antimicrobial peptides. J Biol Chem 279:13896–13901
Paterson DJ, Tassieri M, Reboud J, Wilson R, Cooper JM (2017) Lipid topology and electrostatic interactions underpin lytic activity of linear cationic antimicrobial peptides in membranes. Proc Natl Acad Sci 114:E8324–E8332
Phyu S et al (2003) Drug-Resistant Mycobacterium tuberculosis among new tuberculosis patients, Yangon, Myanmar. Emerg Infect Dis 9:274–276
Podinovskaia M, Lee W, Caldwell S, Russell DG (2013) Infection of macrophages with Mycobacterium tuberculosis induces global modifications to phagosomal function. Cell Microbiol 15:843–859
Ravichandran G, Kumaresan V, Arasu MV, Al-Dhabi NA, Ganesh M-R, Mahesh A, Dhayalan A, Pasupuleti M, Arockiaraj J (2016) Pellino-1 derived cationic antimicrobial prawn peptide: bactericidal activity, toxicity and mode of action. Mol Immunol 78:171–182
Rueda J, Realpe T, Mejia G, Zapata E, Robledo J (2015) GenoType MTBDR plus 1.0(R) for the detection of cross-resistance between isoniazide and ethionamide in isolates of multidrug-resistant Mycobacterium tuberculosis. Biomedica 35:541–548
Saikia K, Sravani YD, Ramakrishnan V, Chaudhary N (2017) Highly potent antimicrobial peptides from N-terminal membrane-binding region of E. coli MreB. Sci Rep 7:42994
Sandhu G (2011) Tuberculosis: current situation, challenges and overview of its control program in India. J Global Infect Dis 3:143–150
Shai Y (2002) Mode of action of membrane active antimicrobial peptides. Biopolymers 66:236–248
Shai Y, Oren Z (1996) Diastereomers of cytolysins, a novel class of potent antibacterial peptides. J Biol Chem 271:7305–7308
Sommer MOA, Munck C, Toft-Kehler RV, Andersson DI (2017) Prediction of antibiotic resistance: time for a new preclinical paradigm? Nat Rev Microbiol 15:689
Starr CG, Wimley WC (2017) Antimicrobial peptides are degraded by the cytosolic proteases of human erythrocytes. Biochim Biophys Acta Biomembr 1859:2319–2326
Stefanini ACB, da Cunha BR, Henrique T, Tajara EH (2015) Involvement of Kallikrein-related peptidases in normal and pathologic processes. Dis Markers 2015:946572
Verreck FAW et al (2017) Variable BCG efficacy in rhesus populations: Pulmonary BCG provides protection where standard intra-dermal vaccination fails. Tuberculosis 104:46–45
Wimley WC (2010) Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem Biol 5:905–917
Xu W, Zhu X, Tan T, Li W, Shan A (2014) Design of embedded-hybrid antimicrobial peptides with enhanced cell selectivity and anti-biofilm activity. PLoS ONE 9:e98935
Yeaman MR, Yount NY (2003) Mechanisms of antimicrobial peptide action and resistance. Pharmacogn Rev 55:27–55
Zuniga ES, Early J, Parish T (2015) The future for early-stage tuberculosis drug discovery. Future Microbiol 10:217–229
Acknowledgements
Authors acknowledge Central Instrument Facility, IIT Guwahati for analytical support. Patent filed and published for antimicrobial peptide (Patent No: 333/KOL/2015 dated 26/03/2015).
Funding
This study was funded by BRNS (Project No. BSBESPNBRNS00864xxVR006), Govt. of India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Ethical Approval
All the testing and experiment related to human blood was performed as per the norms of ethical guidelines approved by ethical committee of Indian Institute of Technology Guwahati.
Informed Consent
All the subjects were informed and an informed consent was obtained as per the requirement.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hazam, P.K., Singh, A., Chaudhary, N. et al. Bactericidal Potency and Extended Serum Life of Stereo-Chemically Engineered Peptides Against Mycobacterium. Int J Pept Res Ther 25, 465–472 (2019). https://doi.org/10.1007/s10989-018-9690-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-018-9690-0