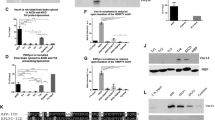
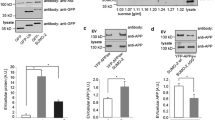
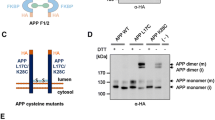
Abstract
Cellular protein phosphorylation regulates proteolytic processing of the Alzheimer’s Amyloid Precursor Protein (APP). This appears to occur both indirectly and directly via APP phosphorylation at residues within cytoplasmic motifs related to targeting and protein–protein interactions. The sorting signal 653YTSI656 comprises the S655 residue that can be phosphorylated by PKC, particularly in mature APP molecules. The YTSI domain has been associated with APP internalization and Golgi polarized sorting, but no functional significance has been attributed to S655 phosphorylation thus far. Using APP695-GFP S655 phosphomutants we show that S655 phosphorylation is a signal that positively modulates APP secretory traffic. The phosphomimicking and dephosphomimicking S655 mutants exhibited contrasting Golgi dynamics, which correlated with differential Golgi vesicular exit and secretory cleavage to sAPP. The role of S655 phosphorylation in APP trafficking at sorting stations, such as the Golgi, its contribution toward cytoprotective alpha sAPP production, and implications for Alzheimer’s disease are discussed.




Similar content being viewed by others
Abbreviations
- p/s:
-
Penicillin/streptomycin
- AD:
-
Alzheimer’s disease
- AICD:
-
APP intracellular domain
- APP:
-
Amyloid precursor protein
- CHX:
-
Cycloheximide
- GFP:
-
Green fluorescence protein
References
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297:353–356. doi:10.1126/science.1072994
Lee VM-Y (2001) Tauists and Baptists united—well almost!. Science 293:1446–1447. doi:10.1126/science.1064684
Haass C, Schlossmacher MG, Hung AY, Vigo-Pelfrey C, Mellon A, Ostaszewski BL, Lieberburg I, Koo EH, Schenk D, Teplow DB, Selkoe DJ (1992) Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature 359:322–325. doi:10.1038/359322a0
Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schlossmacher M, Whaley J, Swindlehurst C, McCormack R, Wolfert R, Selkoe D, Lieberburg I, Schenk D (1992) Isolation and quantification of soluble Alzheimer’s beta-peptide from biological fluids. Nature 359:325–327. doi:10.1038/359325a0
Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R et al (1999) Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286:735–741. doi:10.1126/science.286.5440.735
Turner PR, O’Connor K, Tate WP, Abraham WC (2003) Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog Neurobiol 70:1–32. doi:10.1016/S0301-0082(03)00089-3
Kaether C, Haass C, Steiner H (2006) Assembly, trafficking and function of gamma-secretase. Neurodegener Dis 3:275–283. doi:10.1159/000095267
Sato T, Diehl TS, Narayanan S, Funamoto S, Ihara Y, De Strooper B, Steiner H, Haass C, Wolfe MS (2007) Active gamma-secretase complexes contain only one of each component. J Biol Chem 282:33985–33993. doi:10.1074/jbc.M705248200
Selkoe DJ, Yamazaki M, Citron M, Podlisny MB, Koo EH, Teplow DB, Haass C (1996) The role of APP processing and trafficking pathways in the formation of amyloid β-protein. Ann N Y Acad Sci 777:57–64. doi:10.1111/j.1749-6632.1996.tb34401.x
Gouras GK (2001) Current theories for the molecular and cellular pathogenesis of the Alzheimer’s disease. Expert Rev Mol Med 31:1–11
Small SA, Gandy S (2006) Sorting through the cell biology of Alzheimer’s disease: intracellular pathways to pathogenesis. Neuron 52:15–31. doi:10.1016/j.neuron.2006.09.001
Grbovic OM, Mathews PM, Jiang Y, Schmidt SD, Dinakar R, Summers-Terio NB, Ceresa BP, Nixon RA, Cataldo AM (2003) Rab5-stimulated up-regulation of the endocytic pathway increases intracellular beta-cleaved amyloid precursor protein carboxyl-terminal fragment levels and Abeta production. J Biol Chem 278:31261–31268. doi:10.1074/jbc.M304122200
Yan R, Han P, Miao H, Greengard P, Xu H (2001) The transmembrane domain of the Alzheimer’s beta-secretase (BACE1) determines its late Golgi localization and access to beta-amyloid precursor protein (APP) substrate. J Biol Chem 276:36788–36796. doi:10.1074/jbc.M104350200
Allinson TM, Parkin ET, Turner AJ, Hooper NM (2003) ADAMs family members as amyloid precursor protein alpha-secretases. J Neurosci Res 74:342–352. doi:10.1002/jnr.10737
Koo EH, Squazzo SL, Selkoe DJ, Koo CH (1996) Trafficking of cell-surface amyloid beta-protein precursor I secretion, endocytosis and recycling as detected by labeled monoclonal antibody. J Cell Sci 109:991–998
Yamazaki T, Koo EH, Selkoe DJ (1996) Trafficking of cell-surface beta-amyloid protein precursor II Endocytosis, recycling, and lysosomal targeting detected by immunolocalization. J Cell Sci 109:999–1008
Kuentzel SL, Ali SM, Altman RA, Greenberg BD, Raub TJ (1993) The Alzheimer beta-amyloid protein precursor/protease nexin-II is cleaved by secretase in a trans-Golgi secretory compartment in human neuroglioma cells. Biochem J 295:367–378
Marambaud P, Lopez-Perez E, Wilk S, Checler F (1997) Constitutive and protein kinase C-regulated secretory cleavage of Alzheimer’s beta-amyloid precursor protein: different control of early and late events by the proteasome. J Neurochem 69:2500–2505
Khvotchev M, Südhof TC (2004) Proteolytic processing of amyloid-beta precursor protein by secretases does not require cell surface transport. J Biol Chem 279:47101–47108. doi:10.1074/jbc.M408474200
Caporaso GL, Gandy SE, Buxbaum JD, Ramabhradan TV, Greengard P (1992) Protein phosphorylation regulates secretion of Alzheimer β/A4 amyloid precursor protein. Proc Natl Acad Sci USA 89:3055–3059. doi:10.1073/pnas.89.7.3055
Gillespie SL, Golde TE, Younkin SG (1992) Secretory processing of the Alzheimer amyloid β/A4 protein precursor is increased by protein phosphorylation. Biochem Biophys Res Commun 187:1285–1290. doi:10.1016/0006-291X(92)90442-N
da Cruz e Silva EF, da Cruz e Silva OA, Zaia CT, Greengard P (1995) Inhibition of protein phosphatase 1 stimulates secretion of Alzheimer amyloid precursor protein. Mol Med 1:535–541. doi:10.1007/BF01565937
Ando K, Iijima KI, Elliott JI, Kirino Y, Suzuki T (2001) Phosphorylation-dependent regulation of the interaction of amyloid precursor protein with Fe65 affects the production of beta-amyloid. J Biol Chem 276:40353–40361. doi:10.1074/jbc.M106460200
da Cruz e Silva OA, Fardilha M, Henriques AG, Rebelo S, Vieira S, da Cruz e Silva EF (2004) Signal transduction therapeutics: relevance for Alzheimer’s disease. J Mol Neurosci 23:123–142. doi:10.1385/JMN:23:1-2:123
Henriques AG, Vieira SI, Rebelo S, Domingues SCTS, da Cruz e Silva EF, da Cruz e Silva OAB (2007) Isoform specific amyloid-beta protein precursor metabolism. J Alzheimers Dis 11:85–95
Rebelo S, Vieira SI, Esselmann H, Wiltfang J, da Cruz e Silva EF, da Cruz e Silva AO (2007) Tyr687 dependent APP endocytosis and Abeta production. J Mol Neurosci 32:1–8. doi:10.1007/s12031-007-0001-z
Rebelo S, Vieira SI, Esselmann H, Wiltfang J, da Cruz e Silva EF, da Cruz e Silva AO (2007) Tyrosine 687 phosphorylated Alzheimer’s amyloid precursor protein is retained intracellularly and exhibits a decreased turnover rate. Neurodegener Dis 4:78–87. doi:10.1159/000101831
Matsushima H, Shimohama S, Chachin M, Taniguchi T, Kimura J (1996) Ca2+-dependent and Ca2+-independent protein kinase C changes in the brain of patients with Alzheimer’s disease. J Neurochem 67:317–323
Alonso A, Zaidi T, Novak M, Grundke-Iqbal I, Iqbal K (2001) Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci USA 98:6923–6928. doi:10.1073/pnas.121119298
Wang J, Tung YC, Wang Y, Li XT, Iqbal K, Grundke-Iqbal I (2001) Hyperphosphorylation and accumulation of neurofilament proteins in Alzheimer disease brain and in okadaic acid-treated SY5Y cells. FEBS Lett 507:81–87. doi:10.1016/S0014-5793(01)02944-1
Lee M-S, Kao S-C, Lemere CA, Xia W, Tseng H-C, Zhou Y, Neve R, Ahlijanian MK, Tsai L-H (2003) APP processing is regulated by cytoplasmic phosphorylation. J Cell Biol 163:83–95. doi:10.1083/jcb.200301115
Kimberly WT, Zheng JB, Town T, Flavell RA, Selkoe DJ (2005) Physiological regulation of the beta-amyloid precursor protein signaling domain by c-Jun N-terminal kinase JNK3 during neuronal differentiation. J Neurosci 25:5533–5543. doi:10.1523/JNEUROSCI.4883-04.2005
Muresan Z, Muresan V (2007) The amyloid-beta precursor protein is phosphorylated via distinct pathways during differentiation, mitosis, stress, and degeneration. Mol Biol Cell 18:3835–3844. doi:10.1091/mbc.E06-07-0625
Oishi M, Nairn AC, Czernik AJ, Lim GS, Isohara T, Gandy SE, Greengard P, Suzuki T (1997) The cytoplasmic domain of Alzheimer’s amyloid precursor protein is phosphorylated at Thr654, S655 and Thr668 in adult rat brain and cultured cells. Mol Med 3:111–123
Isohara T, Horiuchi A, Watanabe T, Ando K, Czernik AJ, Uno I, Greengard P, Nairn AC, Suzuki T (1999) Phosphorylation of the cytoplasmic domain of Alzheimer’s beta-amyloid precursor protein at Ser655 by a novel protein kinase. Biochem Biophys Res Commun 258:300–305. doi:10.1006/bbrc.1999.0637
Suzuki T, Nairn AC, Gandy SE, Greengard P (1992) Phosphorylation of Alzheimer amyloid precursor protein by protein kinase C. Neuroscience 48:755–761. doi:10.1016/0306-4522(92)90264-3
Haass C, Koo EH, Capell A, Teplow DB, Selkoe DJ (1995) Polarized sorting of beta-amyloid precursor protein and its proteolytic products in MDCK cells is regulated by two independent signals. J Cell Biol 128:537–547. doi:10.1083/jcb.128.4.537
Lai A, Sisodia SS, Trowbridge IS (1995) Characterization of sorting signals in the beta-amyloid precursor protein cytoplasmic domain. J Biol Chem 270:3565–3573. doi:10.1074/jbc.270.8.3565
Lai A, Gibson A, Hopkins CR, Trowbridge IS (1998) Signal-dependent trafficking of beta-amyloid precursor protein-transferrin receptor chimeras in madin-darby canine kidney cells. J Biol Chem 273:3732–3739. doi:10.1074/jbc.273.6.3732
Zheng P, Eastman J, Vande Pol S, Pimplikar SW (1998) PAT1, a microtubule-interacting protein, recognizes the basolateral sorting signal of amyloid precursor protein. Proc Natl Acad Sci USA 95:14745–14750. doi:10.1073/pnas.95.25.14745
Icking A, Amaddii M, Ruonala M, Höning S, Tikkanen R (2007) Polarized transport of Alzheimer amyloid precursor protein is mediated by adaptor protein complex AP1-1B. Traffic 8:285–296. doi:10.1111/j.1600-0854.2006.00526.x
Gough NR, Zweifel ME, Martinez-Augustin O, Aguilar RC, Bonifacino JS, Fambrough DM (1999) Utilization of the indirect lysosome targeting pathway by lysosome-associated membrane proteins (LAMPs) is influenced largely by the C-terminal residue of their GYXXphi targeting signals. J Cell Sci 112:4257–4269
Reaves BJ, Banting G, Luzio JP (1998) Lumenal and transmembrane domains play a role in sorting type I membrane proteins on endocytic pathways. Mol Biol Cell 9:1107–1122
Canuel M, Lefrancois S, Zeng J, Morales CR (2008) AP-1 and retromer play opposite roles in the trafficking of sortilin between the Golgi apparatus and the lysosomes. Biochem Biophys Res Commun 366:724–730. doi:10.1016/j.bbrc.2007.12.015
da Cruz e Silva OA, Iverfeldt K, Oltersdorf T, Sinha S, Lieberburg I, Ramabhadran TV, Suzuki T, Sisodia SS, Gandy S, Greengard P (1993) Regulated cleavage of Alzheimer beta-amyloid precursor protein in the absence of the cytoplasmic tail. Neuroscience 57:873–877. doi:10.1016/0306-4522(93)90031-A
da Cruz e Silva OA, Vieira SI, Rebelo S, da Cruz e Silva EF (2004) A model system to study intracellular trafficking and processing of the Alzheimer’s amyloid precursor protein. Neurodegener Dis 1:196–204. doi:10.1159/000080986
Xu H, Greengard P, Gandy S (1995) Regulated formation of Golgi secretory vesicles containing Alzheimer beta-amyloid precursor protein. J Biol Chem 270:23243–23245. doi:10.1074/jbc.270.40.23243
Simon JP, Ivanov IE, Adesnik M, Sabatini DD (1996) The production of post-Golgi vesicles requires a protein kinase C-like molecule, but not its phosphorylating activity. J Cell Biol 135:355–370. doi:10.1083/jcb.135.2.355
Walter J, Capell A, Hung AY, Langen H, Schnölzer M, Thinakaran G, Sisodia SS, Selkoe DJ, Haass C (1997) Ectodomain phosphorylation of beta-amyloid precursor protein at two distinct cellular locations. J Biol Chem 272:1896–1903. doi:10.1074/jbc.272.45.28582
Henriques A, Vieira SI, Crespo-López E, Oliveira M, da Cruz e Silva EF, da Cruz e Silva OAB (2008) Intracellular sAPPalpha retention in response to Abeta is mapped to cytoskeleton associated structure. J Neurosci Res 87: 1449–1461
Kuismanen E, Saraste J (1989) Low temperature-induced transport blocks as tools to manipulate membrane traffic. Methods Cell Biol 32:257–274. doi:10.1016/S0091-679X(08)61174-7
Ellinger I, Klapper H, Courtoy PJ, Vaerman JP, Fuchs R (2002) Different temperature sensitivity of endosomes involved in transport to lysosomes and transcytosis in rat hepatocytes: analysis by free-flow electrophoresis. Electrophoresis 23:2117–2129. doi:10.1002/1522-2683(200207)23:13<2117::AID-ELPS2117>3.0.CO;2-Z
Furukawa K, Sopher BL, Rydel RE, Begley JG, Pham DG, Martin GM, Fox M, Mattson MP (1996) Increased activity-regulating and neuroprotective efficacy of alpha-secretase-derived secreted amyloid precursor protein conferred by a C-terminal heparin-binding domain. J Neurochem 67:1882–1896
Colciaghi F, Borroni B, Pastorino L, Marcello E, Zimmermann M, Cattabeni F, Padovani A, Di Luca M (2002) [alpha]-Secretase ADAM10 as well as [alpha]APPs is reduced in platelets and CSF of Alzheimer disease patients. Mol Med 8:67–74
Bibl M, Mollenhauer B, Esselmann H, Lewczuk P, Klafki HW, Sparbier K, Smirnov A, Cepek L, Trenkwalder C, Rüther E, Kornhuber J, Otto M, Wiltfang J (2006) CSF amyloid-beta-peptides in Alzheimer’s disease, dementia with Lewy bodies and Parkinson’s disease dementia. Brain 129:1177–1187. doi:10.1093/brain/awl063
Buxbaum JD, Koo EH, Greengard P (1993) Protein phosphorylation inhibits production of Alzheimer amyloid beta/A4 peptide. Proc Natl Acad Sci USA 19:9195–9198. doi:10.1073/pnas.90.19.9195
da Cruz e Silva OAB, Rebelo S, Vieira SI, Gandy S, da Cruz e Silva EF, Greengard P (2008) Enhanced generation of Alzheimer’s amyloid-β following chronic exposure to phorbol ester correlates with differential effects on alpha and epsilon isozymes of protein kinase C. J Neurochem 108:319–330. doi:10.1111/j.1471-4159.2008.05770.x
Muresan Z, Muresan V (2005) Coordinated transport of phosphorylated amyloid-beta precursor protein and c-Jun NH2-terminal kinase-interacting protein-1. J Cell Biol 171:615–625. doi:10.1083/jcb.200502043
Natsume W, Tanabe K, Kon S, Yoshida N, Watanabe T, Torii T, Satake M (2006) SMAP2, a novel ARF GTPase-activating protein, interacts with clathrin and clathrin assembly protein and functions on the AP-1-positive early endosome/trans-Golgi network. Mol Biol Cell 17:2592–2603. doi:10.1091/mbc.E05-10-0909
Seaman MN (2007) Identification of a novel conserved sorting motif required for retromer-mediated endosome-to-TGN retrieval. J Cell Sci 120:2378–2389. doi:10.1242/jcs.009654
Ramelot TA, Nicholson LK (2001) Phosphorylation-induced structural changes in the amyloid precursor protein cytoplasmic tail detected by NMR. J Mol Biol 307:871–884. doi:10.1006/jmbi.2001.4535
Rogaeva E, Meng Y, Lee JH, Gu Y, Kawarai T, Zou F, Katayama T, Baldwin CT, Cheng R, Hasegawa H, Chen F, Shibata N, Lunetta KL, Pardossi-Piquard R, Bohm C et al (2007) The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet 39:168–177. doi:10.1038/ng1943
Sager KL, Wuu J, Leurgans SE, Rees HD, Gearing M, Mufson EJ, Levey AI, Lah JJ (2007) Neuronal LR11/sorLA expression is reduced in mild cognitive impairment. Ann Neurol 62:640–647. doi:10.1002/ana.21190
Dodson SE, Gearing M, Lippa CF, Montine TJ, Levey AI, Lah JJ (2006) LR11/SorLA expression is reduced in sporadic Alzheimer disease but not in familial Alzheimer disease. J Neuropathol Exp Neurol 65:866–872. doi:10.1097/01.jnen.0000228205.19915.20
Muhammad A, Flores I, Zhang H, Yu R, Staniszewski A, Planel E, Herman M, Ho L, Kreber R, Honig LS, Ganetzky B, Duff K, Arancio O, Small SA (2008) Retromer deficiency observed in Alzheimer’s disease causes hippocampal dysfunction, neurodegeneration, and Abeta accumulation. Proc Natl Acad Sci USA 105:7327–7332. doi:10.1073/pnas.0802545105
Acknowledgments
This work was supported by the European Union V and VI Framework Program of the European Union (DIADEM and APOPIS), and by grants from the Fundação para a Ciência e a Tecnologia of the Portuguese Ministry of Sciences and Tecnhology (Projects POCTI/NSE/40682/2001 and POCI/58469/2004 and REEQ/1023/BIO/2005), from the Fundação Calouste Gulbenkian (prémio Estímulo à Investigação, 2003), and from the Centro de Biologia Celular, Universidade de Aveiro. SIV is recipient of a FCT fellowship (SFRH/BPD/19515/2004).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vieira, S.I., Rebelo, S., Domingues, S.C. et al. S655 phosphorylation enhances APP secretory traffic. Mol Cell Biochem 328, 145–154 (2009). https://doi.org/10.1007/s11010-009-0084-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-009-0084-7