Abstract
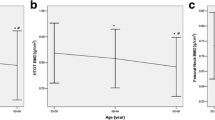
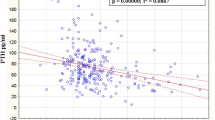
Osteoporosis is a degenerative disease of the skeletal system, and its major complication is fracture that severely influences the living quality of the middle-aged and the aged. The purpose of this study was to investigate the significance of sex hormones and some biochemical indicators related to bone metabolism in the genesis and development of osteoporosis. The plasma samples were collected from 244 post-menopausal women of Xi’an urban area, and their plasma contents of testosterone, estradiol, calcitonin, osteocalcin and N-terminal propeptide of type I procollagen were detected by ELISA. The activity of tartrate-resistant acid phosphatase was determined by spectrophotometric method, and the content of nitric oxide was measured by Griess method. Bone mineral density (BMD) in lumbar vertebrae (L1–L4) and hips was measured by QDR-2000 dual energy X-ray absorptiometry. The concentrations of the biochemical indicators were compared among the three groups (normal bone mass group, osteopenia group and osteoporosis group), and Pearson correlation analysis was used to verify the correlations between the indicators and BMD. The comparison results of blood biochemical indicators of BMD-based groups showed that the plasma contents of estradiol (P = 0.006), testosterone (P = 0.038) and calcitonin (P = 0.042) decreased more significantly in the osteoporosis group, but the content of osteocalcin (P = 0.008) increased significantly in osteoporosis group than those in the other groups. The correlation analysis between BMD of different parts and the blood biochemical indicators showed that there was a significant positive correlation between estradiol and the BMD of lumber vertebra (r = 0.200, P = 0.002), femoral neck (r = 0.160, P = 0.013), and great trochanter (r = 0.204, P = 0.001). Significant positive correlations between calcitonin and BMD of lumber vertebra (r = 0.166, P = 0.018) and femoral great trochanter (r = 0.152, P = 0.041), and between testosterone and BMD of femoral great trochanter (r = 0.158, P = 0.014) were also observed. In addition, there existed significant negative correlations between osteocalcin and BMD of lumber vertebra (r = −0.220, P = 0.001), femoral neck (r = −0.259, P < 0.000), and great trochanter (r = −0.221, P = 0.001), and between the activity of tartrate-resistant acid phosphatase and BMD of femoral great trochanter (r = −0.135, P = 0.037). The partial correlation analysis also showed that there were significant correlations between estradiol (r = 0.160, P = 0.014), calcitonin (r = 0.240, P = 0.013), osteocalcin (r = −0.226, P = 0.023) and BMD when the influence of age was excluded. The Pearson correlation analysis of biochemical indicators showed there were positive correlations between the contents of testosterone and calcitonin, testosterone and osteocalcin, calcitonin and osteocalcin, calcitonin and PINP, calcitonin and NO, osteocalcin and NO, and PINP and NO, but negative correlations between the contents of testosterone and PINP, estradiol and calcitonin, estradiol and osteocalcin, and estradiol and NO. The blood contents of sex hormones and calcitonin significantly influence BMD and osteoporosis development, and the increase of osteocalcin contents could be used as a biomarker to indicate the degree of osteoporosis in post-menopausal women.
Similar content being viewed by others
Abbreviations
- BMD:
-
Bone mineral density
- CT:
-
Calcitonin
- DEXA:
-
Dual energy X-ray absorptiometry
- E2 :
-
Estradiol
- ELISA:
-
Enzyme linked immunosorbent assay
- NO:
-
Nitric oxide
- OC:
-
Osteocalcin
- OD:
-
Optical density
- OP:
-
Osteoporosis
- PINP:
-
N-terminal propeptide of type I procollagen
- TRAP:
-
Tartrate-resistant acid phosphatase
- T:
-
Testosterone
References
Lewiecki EM (2008) Prevention and treatment of postmenopausal osteoporosis. Obstet Gynecol Clin North Am 35:301–315. doi:10.1016/j.ogc.2008.03.007
Walker J (2008) Osteoporosis: pathogenesis, diagnosis and management. Nurs Stand 22:48–56
Li H, Xie H, Liu W, Hu R, Huang B, Tan YF, Xu K, Sheng ZF, Zhou HD, Wu XP, Luo XH (2009) A novel microRNA targeting HDAC5 regulates osteoblast differentiation in mice and contributes to primary osteoporosis in humans. J Clin Invest 119:3666–3677
Falcón-Ramírez E, Casas-Avila L, Miranda A, Diez P, Castro C, Rubio J, Gómez R, Valdés-Flores M (2010) Sp1 polymorphism in collagen I alpha1 gene is associated with osteoporosis in lumbar spine of Mexican women. Mol Biol Rep. doi:10.1007/s11033-010-9963-y
Lee YH, Woo JH, Choi SJ, Ji JD, Song GG (2010) Associations between osteoprotegerin polymorphisms and bone mineral density: a meta-analysis. Mol Biol Rep 37(1):227–234
Ilich JZ, Kerstetter JE (2000) Nutrition in bone health revisited: a story beyond calcium. J Am Coll Nutr 19:715–737
Palacios C (2006) The role of nutrients in bone health, from A to Z. Crit Rev Food Sci Nutr 46:621–628. doi:10.1080/10408390500466174
Prentice A (2004) Diet, nutrition and the prevention of osteoporosis. Public Health Nutr 7:227–243. doi:10.1079/PHN2003590
Manios Y, Moschonis G, Trovas G, Lyritis GP (2007) Changes in biochemical indexes of bone metabolism and bone mineral density after a 12-mo dietary intervention program: the Postmenopausal Health Study. Am J Clin Nutr 86:781–789
Williams FM, Spector TD (2007) The genetics of osteoporosis. Acta Reumatol Port 32:231–240
Haas ML, Moore K (2007) Osteoporosis: an invisible, undertreated, and neglected disease of elderly men. J Elder Abuse Negl 19:61–73
Liu SZ, Yan H, Xu P, Hou B, Zhuang GH, Zeng YH, Guo X, Shemin Lu (2008) Correlational analysis between bone mineral density and physiological characters of postmenopausal women in Xi’an urban area. J Xi’an Jiaotong Univ (Med Sci) 29:107–109 [in Chinese]
Liu Z, Piao J, Pang L, Qing X, Nan S, Pan Z, Guo Y, Wang X, Li F, Liu J, Cheng X (2002) The diagnostic criteria for primary osteoporosis and the incidence of osteoporosis in China. J Bone Miner Metab 20:181–189
Mutlu M, Argun M, Kilic E, Saraymen R, Yazar S (2007) Magnesium, zinc and copper status in osteoporotic, osteopenic and normal post-menopausal women. J Int Med Res 35:692–695
Imashuku Y, Takada M, Murata K (2007) Comparisons of bone mass measurements on various skeletal sites including quantitative ultrasonography of the calcaneus for assessing age-related losses, their correlations, and diagnostic agreement using the Japanese and WHO criteria for osteoporosis. Radiat Med 25:148–154. doi:10.1007/s11604-006-0117-z
Miller PD (2006) Guidelines for the diagnosis of osteoporosis: T-scores vs fractures. Rev Endocr Metab Disord 7:75–89. doi:10.1007/s11154-006-9006-0
Frank GR (2003) Role of estrogen and androgen in pubertal skeletal physiology. Med Pediatr Oncol 41:217–221. doi:10.1002/mpo.10340 PMID: 12868122
Alexandre C (2005) Androgens and bone metabolism. Joint Bone Spine 72:202–206. doi:10.1016/j.jbspin.2004.04.004
Deng X, Wang W, Wu X, Huang G, Peng J, Liao E, Wu H (2000) Correlation between bone mineral density and sexual hormones in healthy Chinese women. J Environ Pathol Toxicol Oncol 19:167–169
Riggs BL, Khosla S, Atkinson EJ, Dunstan CR, Melton LJ III (2003) Evidence that type I osteoporosis results from enhanced responsiveness of bone to estrogen deficiency. Osteoporos Int 14:728–733. doi:10.1007/s00198-003-1437-9
Tok EC, Ertunc D, Oz U, Camdeviren H, Ozdemir G, Dilek S (2004) The effect of circulating androgens on bone mineral density in postmenopausal women. Maturitas 48:235–242. doi:10.1016/j.maturitas.2003.11.007
Zofková I, Bahbouh R, Hill M (2000) The pathophysiological implications of circulating androgens on bone mineral density in a normal female population. Steroids 65:857–861. doi:10.1016/S0039-128X(00)00136-7
Gambacciani M, Vacca F (2004) Postmenopausal osteoporosis and hormone replacement therapy. Minerva Med 95:507–520
Riggs BL (2002) Endocrine causes of age-related bone loss and osteoporosis. Novartis Found Symp 242:247–259
Lerner UH (2006) Bone remodeling in post-menopausal osteoporosis. J Dent Res 85:584–595
Rapuri PB, Gallagher JC, Haynatzki G (2004) Endogenous levels of serum estradiol and sex hormone binding globulin determine bone mineral density, bone remodeling, the rate of bone loss, and response to treatment with estrogen in elderly women. J Clin Endocrinol Metab 89:4954–4962
Kamel HK, Perry HM III, Morley JE (2001) Hormone replacement therapy and fractures in older adults. J Am Geriatr Soc 49:179–187. doi:10.1046/j.1532-5415.2001.49040.x
Whitehead M (2006) Hormone replacement therapy with estradiol and drospirenone: an overview of the clinical data. J Br Menopause Soc 12(Suppl 1):4–7
Naot D, Cornish J (2008) The role of peptides and receptors of the calcitonin family in the regulation of bone metabolism. Bone 43:813–818. doi:10.1016/j.bone.2008.07.003
Huebner AK, Keller J, Catala-Lehnen P, Perkovic S, Streichert T, Emeson RB, Amling M, Schinke T (2008) The role of calcitonin and alpha-calcitonin gene-related peptide in bone formation. Arch Biochem Biophys 473:210–217. doi:10.1016/j.abb.2008.02.013
Ozgocmen S, Kaya H, Fadillioglu E, Yilmaz Z (2007) Effects of calcitonin, risedronate, and raloxifene on erythrocyte antioxidant enzyme activity, lipid peroxidation, and nitric oxide in postmenopausal osteoporosis. Arch Med Res 38:196–205. doi:10.1016/j.arcmed.2006.09.010
Chesnut CH III, Azria M, Silverman S, Engelhardt M, Olson M, Mindeholm L (2008) Salmon calcitonin: a review of current and future therapeutic indications. Osteoporos Int 19:479–491. doi:10.1007/s00198-007-0490-1
Muñoz-Torres M, Alonso G, Raya MP (2004) Calcitonin therapy in osteoporosis. Treat Endocrinol 3:117–132
Mehta NM, Malootian A, Gilligan JP (2003) Calcitonin for osteoporosis and bone pain. Curr Pharm Des 9:2659–2676
Tekeoğlu I, Adak B, Budancamanak M, Demirel A, Ediz L (2005) Comparison of cyclic and continuous calcitonin regimens in the treatment of postmenopausal osteoporosis. Rheumatol Int 26:157–161. doi:10.1007/s00296-004-0549-7
Lee AJ, Hodges S, Eastell R (2000) Measurement of osteocalcin. Ann Clin Biochem 37(Pt 4):432–446
Ivaska KK, Käkönen SM, Gerdhem P, Obrant KJ, Pettersson K, Väänänen HK (2005) Urinary osteocalcin as a marker of bone metabolism. Clin Chem 51:618–628. doi:10.1373/clinchem.2004.043901
Soontrapa S, Soontrapa S, Bunyaratavej N (2005) Serum concentration of undercarboxylated osteocalcin and the risk of osteoporosis in Thai elderly women. J Med Assoc Thai 88(Suppl 5):S29–S32
Eastell R, Hannon RA (2008) Biomarkers of bone health and osteoporosis risk. Proc Nutr Soc 67:157–162. doi:10.1017/S002966510800699X
Adami S, Bianchi G, Brandi ML, Giannini S, Ortolani S, DiMunno O, Frediani B, Rossini M, BONTURNO Study Group (2008) Determinants of bone turnover markers in healthy premenopausal women. Calcif Tissue Int 82:341–347. doi:10.1007/s00223-008-9126-5
Desai MP, Bhanuprakash KV, Khatkhatay MI, Donde UM (2007) Age-related changes in bone turnover markers and ovarian hormones in premenopausal and postmenopausal Indian women. J Clin Lab Anal 21:55–60. doi:10.1002/jcla.20166
Ljusberg J, Wang Y, Lång P, Norgård M, Dodds R, Hultenby K, Ek-Rylander B, Andersson G (2005) Proteolytic excision of a repressive loop domain in tartrate-resistant acid phosphatase by cathepsin K in osteoclasts. J Biol Chem 280:28370–28381. doi:10.1074/jbc.M502469200
Halleen JM (2003) Tartrate-resistant acid phosphatase 5B is a specific and sensitive marker of bone resorption. Anticancer Res 23(2A):1027–1029
Andersson G, EK-Rylander B (1995) The tartrate-resistant purple acid phosphatase of bone osteoclasts. a protein phosphatase with multivalent substrate specificity and regulation. Acta Orthop Scand 66(Suppl):189–190
Qin YJ, Zhang ZL, Zhang H, Hu WW, Liu YJ, Hu YQ, Li M, Gu JM, He JW (2008) Age-related changes of serum tartrate-resistant acid phosphatase 5b and the relationship with bone mineral density in Chinese women. Acta Pharmacol Sin 29:1493–1498. doi:10.1111/j.1745-7254.2008.00890.x
Reginster JY, Sarkar S, Zegels B, Henrotin Y, Bruyere O, Agnusdei D, Collette J (2004) Reduction in PINP, a marker of bone metabolism, with raloxifene treatment and its relationship with vertebral fracture risk. Bone 34:344–351. doi:10.1016/j.bone.2003.10.004
Hale LV, Galvin RJ, Risteli J, Ma YL, Harvey AK, Yang X, Cain RL, Zeng Q, Frolik CA, Sato M, Schmidt AL, Geiser AG (2007) PINP: a serum biomarker of bone formation in the rat. Bone 40:1103–1109. doi:10.1016/j.bone.2006.11.027
Hari Kumar KV, Muthukrishnan J, Verma A, Modi KD (2008) Correlation between bone markers and bone mineral density in postmenopausal women with osteoporosis. Endocr Pract 14:1102–1107
Mostaza JM, De la Piedra C, Curiel MD, Peña R, Lahoz C (2001) Pravastatin therapy increases procollagen I N-terminal propeptide (PINP), a marker of bone formation in post-menopausal women. Clin Chim Acta 308:133–137. doi:10.1016/S0009-8981(01)00476-4
Brennan PA, Sharma S, Bowden JR, Umar T (2003) Expression of inducible nitric oxide synthase in bone metastases. Eur J Surg Oncol 29:619–623. doi:10.1016/S0748-7983(03)00105-7
Hof RJ, Macphee J, Libouban H, Helfrich MH, Ralston SH (2004) Regulation of bone mass and bone turnover by neuronal nitric oxide synthase. Endocrinology 145:5068–5074. doi:10.1210/en.2004-0205
Hao YJ, Tang Y, Chen FB, Pei FX (2005) Different doses of nitric oxide donor prevent osteoporosis in ovariectomized rats. Clin Orthop Relat Res 435:226–231
Wimalawansa SJ (2008) Nitric oxide: novel therapy for osteoporosis. Expert Opin Pharmacother 9:3025–3044. doi:10.1517/14656560802197162
Hua Zhang, Ganfeng Chen, Junzhe Wu, Yanxian Chen (2008) Significance of Leptin, NO, IL-6, BGP and E2 detected in serum of postmenopausal women with osteoporosis. Chin J Trad Med Traum Orthop 16:1–3 [in Chinese]
Ozgocmen S, Kaya H, Fadillioglu E, Aydogan R, Yilmaz Z (2007) Role of antioxidant systems, lipid peroxidation, and nitric oxide in postmenopausal osteoporosis. Mol Cell Biochem 295:45–52. doi:10.1007/s11010-006-9270-z
Acknowledgements
Sincere thanks to the Health Bureaus of Beilin District, Yanta District,Xincheng District, and Lianhu District, and their community medical clinics in Xi’an for their help in the epidemiologic investigation. The work was supported by Xi’an Municipal Science and Technology Research Project Fund (GG06152), Shaanxi Provincial Science and Technology Research and Development Project Fund (2007K14-01) and the National Natural Science Foundation of China (30630058, 30571725).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, Sz., Tian, Lf., Xu, P. et al. Analysis of correlation between blood biochemical indicators and bone mineral density of post-menopausal women. Mol Biol Rep 38, 939–948 (2011). https://doi.org/10.1007/s11033-010-0187-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-0187-y