Abstract
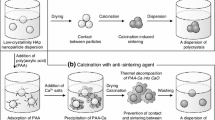
Sintering-free nanocrystals of calcined hydroxyapatite (HAp) having a rod-like morphology were fabricated by calcination at 800°C for 1 h with an anti-sintering agent surrounding original HAp particles and the agent was subsequently removed after calcination. The original HAp particles having a rod-like morphology with a size ranging from 30 to 80 nm (short axis) and 300 to 500 nm (long axis) were prepared by wet chemical process, and poly(acrylic acid, calcium salt) (PAA-Ca) was used as the anti-sintering agent. In the case of calcination without additives, the mean size of HAp crystals dispersed in an ethanol medium increased by about 4 times and the specific surface area of the crystals exhibited a 25% decrease compared to those of the original HAp particles because of calcination-induced sintering among the crystals. On the other hand, the HAp crystals calcined with the anti-sintering agent, PAA-Ca, could be dispersed in an ethanol medium at the same size as the original particles, and they preserved the specific surface area after calcination. These results indicate that PAA-Ca and/or its thermally decomposed product, CaO, surrounded the HAp particles and protected them against calcination-induced sintering during calcination. The HAp crystals calcined with PAA-Ca showed high crystallinity, and no other calcium phosphate phases could be detected after washing with water.
Similar content being viewed by others
References
Aoki H., 1991. Science and Medical Application of Hydroxyapatite. Takayama Press System Center Co., Inc
Barralet J.E., Best S.M. and Bonfield W. (2000) Effect of sintering parameters on the density and microstructure of carbonate hydroxyapatite. J. Mater. Sci. Mater. Med. 11:719–724
Bernache-Assollant D., Ababoua A., Championa E. and Heughebaertb M. (2003) Sintering of calcium phosphate hydroxyapatite Ca10(PO4)6(OH)2 I. Calcination and particle growth. J. Eur. Ceram. Soc. 23: 229–241
Bonapasta A.A., Buda F. and Colombet P. (2001) Interaction between Ca ions and poly(acrylic acid) chains in macro-defect-free cements: A theoretical study. Chem. Mater. 13: 64–70
Carless J.E. and Foster A.A. (1966) Accelerated crystal growth of sulfathiazole by temperature cycling. J. Pharmaceut. Pharmacol. 18: 697–708
Cheng Z.H., Yasukawa A., Kandori K. and Ishikawa T. (1998) FTIR study on incorporation of CO2 into calcium hydroxyapatite. J. Chem. Soc. Faraday Trans. 94: 1501–1505
Cushing B.L., Kolesnichenko V.L. and O’Connor C.J. (2004) Recent advances in the liquid-phase syntheses of inorganic nanoparticles. Chem. Rev. 104: 3893–3946
Emerson W.H. and Fisher E.E. (1962) The infrared absorption spectra of carbonate in calcified tissue. Arch. Oral. Biol. 7: 671–683
Fowler B.O. (1974) Infrared studies of apatites. I Vibrational assignments for calcium, strontium, and barium hydroxyapatites utilizing isotopic substitution. Inorg. Chem. 13: 194–206
Furuzono T., Kishida A. and Tanaka J. (2004) Nano-scaled hydroxyapatite/polymer composite I. Coating of sintered hydroxyapatite particles on poly(γ-methacryloxypropyl trimethoxysilane)-grafted silk fibroin fibers through chemical bonding. J. Mater. Sci. Mater. Med. 15: 19–23
Furuzono T., Sonoda K. and Tanaka J. (2001) A hydroxyapatite coating covalently linked onto a silicone implant material. J. Biomed. Mater. Res. 56: 9–12
Furuzono T., Wang P., Korematsu A., Miyazaki K., Oido-Mori M., Kowashi Y., Ohura K., Tanaka J. and Kishida A. (2003) Physical and biological evaluations of sintered hydroxyapatite/silicone composite with covalent bonding for a percutaneous implant material. J. Biomed. Mater. Res. B: Appl. Biomater. 65B: 217–226
Furuzono T., M. Masuda, M. Okada, S. Yasuda, H. Kadono, R. Tanaka & K. Miyatake, 2006. Increase of cell adhesiveness on poly(ethylene terephthalate) fabric by coating of sintered hydroxyapatite nanocrystals for development of an artificial blood vessel. ASAIO J. (in press)
Gibson I.R. and Bonfield W. (2002) Novel synthesis and characterization of an AB-type carbonate-substituted hydroxyapatite. J. Biomed. Mater. Res. 59: 697–708
Ishikawa K., Ishikawa Y. and Kuwayama N. (1991) Preparation of carbonate-bearing hydroxyapatites and their sintering properties. Chem. Express 6: 463–466
Jarcho M., Bolen C.H., Thomas M.B., Bobick J., Kay J.F. and Doremus R.H. (1976) Hydroxylapatite synthesis and characterization in dense polycrystalline form. J. Mater. Sci. 11: 2027–2035
Kawasaki T. (1991) Hydroxyapatite as a liquid chromatographic packing. J. Chromatogr. 544: 147–184
Landi E., Tampieri A., Celotti G. and Sprio S. (2000) Densification behaviour and mechanisms of synthetic hydroxyapatites. J. Eur. Ceram. Soc. 20: 2377–2387
Lim G.K., Wang J., Ng S.C. and Gan L.M. (1996) Processing of fine hydroxyapatite powders via an inverse microemulsion route. Mater. Lett. 28: 431–436
Masuda Y., Matsubara K. and Sakka S. (1990) Synthesis of hydroxyapatite from metal alkoxides through sol–gel technique. J. Ceram. Soc. Jpn. 98: 1255–1266
Misra D.N. (1993) Adsorption of low-molecular-weight sodium polyacrylate on hydroxyapatite. J. Dent. Res. 10: 1418–1422
Okada M. & T. Furuzono, 2006. Nano-sized ceramic particles of hydroxyapatite calcined with an anti-sintering agent. J. Nanosci. Nanotech. (in press)
Papargyris A.D., Botis A.I. and Papargyri S.A. (2002) Synthetic routes for hydroxyapatite powder production. Key. Eng. Mater. 206–213: 83–86
Sanchez C. and Livage J. (1990) Sol–gel chemistry from metal alkoxide precursors. New J. Chem. 14: 513–521
Schmidt H.K. (2000) Nanoparticles for ceramic and nanocomposite processing. Mol. Cryst. Liq. Cryst. 353: 165–179
Schmidt H.K., Geiter E., Mennig M., Krug H., Becker C. and Winkler R.-P. (1998) The sol–gel process for nano-technologies: New nanocomposites with interesting optical and mechanical properties. J. Sol–Gel Sci. Tech. 13: 397–404
Sindhu S. and Valiyaveettil S. (2004) Design and synthesis of optically transparent calcium incorporated polymer complex. J. Polym. Sci. B: Polym. Phys. 42: 4459–4465
Somiya S., Ioku K. and Yoshimura M. (1988) Hydrothermal synthesis and characterization of fine apatite crystals. Mater. Sci. Forum 34–36:371–378
Sonoda K., Furuzono T., Walsh D., Sato K. and Tanaka J. (2002) Influence of emulsion on crystal growth of hydroxyapatite. Solid State Ionics 151:321–327
Suchanek W., Yashima M., Kakihana M., Yoshimura M. (1997) Hydroxyapatite/hydroxyapatite-whisker composites without sintering additives: Mechanical properties and microstructural evolution. J. Am. Ceram. Soc. 80:2805–2813
Taylor M.G., Parker S.F., Simkiss K. and Mitchell P.C.H. (2001) Bone mineral: Evidence for hydroxy groups by inelastic neutron scattering. Phys. Chem. Chem. Phys. 3: 1514–1517
Yoshida Y., Van Meerbeek B., Nakayama Y., Yoshioka M., Snauwaert J., Abe Y., Lambrechts P., Vanherle G. and Okazaki M. (2001) Adhesion to and decalcification of hydroxyapatite by carboxylic acids. J. Dent. Res. 80: 1565–1569
Yoshimura M., Suda H., Okamoto K. and Ioku K. (1994) Hydrothermal synthesis of biocompatible whiskers. J. Mater. Sci. 29: 3399–3402
Zabetakis P.M., Cotell C.M., Chrisey D.B. and Auyeung R.C. (1994) Pulsed laserdeposition of thin film hydroxyapatite. Applications for flexible catheters. ASAIO J. 40: 869–899
Acknowledgment
We thank Dr. Y. Yokogawa and Dr. K. Sato of the Advanced Manufacturing Research Institute, National Institute of Advanced Industrial Science and Technology (AIST), for helpful discussions. This work was supported by a Research Grant for Cardiovascular Diseases from the Ministry of Health, Labour and Welfare, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Okada, M., Furuzono, T. Calcination of Rod-like Hydroxyapatite Nanocrystals with an Anti-sintering Agent Surrounding the Crystals. J Nanopart Res 9, 807–815 (2007). https://doi.org/10.1007/s11051-006-9126-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-006-9126-1