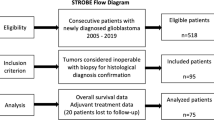
Abstract
Purpose
The prognostic impact of the histopathologic features of recurrent glioblastoma surgical specimens is unknown. We sought to determine whether key histopathologic characteristics in glioblastoma tumors resected after chemoradiotherapy are associated with overall survival (OS).
Methods
The following characteristics were quantified in recurrent glioblastoma specimens at our institution: extent of viable tumor (accounting for % of specimen comprised of tumor and tumor cellularity), mitoses per 10 high-power fields (0, 1–10, > 10), Ki-67 proliferative index (0–100%), hyalinization (0–6; none to extensive), rarefaction (0–6), hemosiderin (0–6), and % of specimen comprised of geographic necrosis (0–100%; converted to 0–6 scale). Variables associated with OS in univariate analysis, as well as age, eastern cooperative oncology group performance status (ECOG PS), extent of repeat resection, time from initial diagnosis to repeat surgery, and O6-methylguanine-DNA methyltransferase promoter methylation, were included in a multivariable Cox proportional hazards model.
Results
37 specimens were assessed. In a multivariate model, high Ki-67 proliferative index was the only histopathologic characteristic associated with worse OS following repeat surgery for glioblastoma (hazard ratio (HR) 1.3, 95% CI 1.1–1.5, p = 0.003). Shorter time interval from initial diagnosis to repeat surgery (HR 1.11, 95% CI 1.02–1.21, p = 0.016) and ECOG PS ≥ 2 (HR 4.19, 95% CI 1.72–10.21, p = 0.002) were also independently associated with inferior OS.
Conclusion
In patients with glioblastoma undergoing repeat resection following chemoradiotherapy, high Ki-67 index in the recurrent specimen, short time to recurrence, and poor PS are independently associated with worse OS. Histopathologic quantification of viable tumor versus therapy-related changes has limited prognostic influence.



Similar content being viewed by others
References
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Cloughesy T, Perry JR, Wick W (2013) Standards of care for treatment of recurrent glioblastoma—are we there yet? Neuro Oncol 15(1):4–27
Weller M, van den Bent M, Tonn JC et al (2017) European association for neuro-oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol 18(6):e315–e329
Montemurro N, Perrini P, Blanco MO, Vannozzi R (2016) Second surgery for recurrent glioblastoma: a concise overview of the current literature. Clin Neurol Neurosurg 142:60–64
Verma N, Cowperthwaite MC, Burnett MG, Markey MK (2013) Differentiating tumor recurrence from treatment necrosis: a review of neuro-oncologic imaging strategies. Neuro-Oncology 15(5):515–534
Burger PC, Mahley MS Jr, Dudka L, Vogel FS (1979) The morphologic effects of radiation administered therapeutically for intracranial gliomas: a postmortem study of 25 cases. Cancer 44(4):1256–1272
Woodworth GF, Garzon-Muvdi T, Ye X, Blakeley JO, Weingart JD, Burger PC (2013) Histopathological correlates with survival in reoperated glioblastomas. J Neurooncol 113(3):485–493
Tihan T, Barletta J, Parney I, Lamborn K, Sneed PK, Chang S (2006) Prognostic value of detecting recurrent glioblastoma multiforme in surgical specimens from patients after radiotherapy: should pathology evaluation alter treatment decisions? Hum Pathol 37(3):272–282
Clarke JL, Chang S (2009) Pseudoprogression and pseudoresponse: challenges in brain tumor imaging. Curr Neurol Neurosci Rep 9(3):241–246
Forsyth PA, Kelly PJ, Cascino TL et al (1995) Radiation necrosis or glioma recurrence: is computer-assisted stereotactic biopsy useful? J Neurosurg 82(3):436–444
McGirt MJ, Bulsara KR, Cummings TJ et al (2003) Prognostic value of magnetic resonance imaging-guided stereotactic biopsy in the evalution of recurrent malignant astrocytoma compared with a lesion due to radiation effect. J Neurosurg 98(1):14–20
van Nifterik KA, van den Berg J, Stalpers LJ et al (2007) Differential radiosensitizing potential of temozolomide in MGMT promoter methylated glioblastoma multiforme cell lines. Int J Radiat Oncol Biol Phys 69(4):1246–1253
Pala A, Schmitz AL, Knoll A et al (2018) Is MGMT promoter methylation to be considered in the decision making for recurrent surgery in glioblastoma patients? Clin Neurol Neurosurg 167:6–10
Audureau E, Chivet A, Ursu R et al (2018) Prognostic factors for survival in adult patients with recurrent glioblastoma: a decision-tree-based model. J Neuro-Oncol 136(3):565–576
Brandes AA, Bartolotti M, Tosoni A et al (2016) Patient outcomes following second surgery for recurrent glioblastoma. Future Oncol (Lond Engl) 12(8):1039–1044
Melguizo-Gavilanes I, Bruner JM, Guha-Thakurta N, Hess KR, Puduvalli VK (2015) Characterization of pseudoprogression in patients with glioblastoma: is histology the gold standard? J Neuro-Oncol 123(1):141–150
Ralte AM, Sharma MC, Karak AK, Mehta VS, Sarkar C (2001) Clinicopathological features, MIB-1 labeling index and apoptotic index in recurrent astrocytic tumors. Pathol Oncol Res 7(4):267–278
Schroder R, Feisel KD, Ernestus RI (2002) Ki-67 labeling is correlated with the time to recurrence in primary glioblastomas. J Neuro-Oncol 56(2):127–132
Kuriyama H, Lamborn KR, O’Fallon JR et al (2002) Prognostic significance of an apoptotic index and apoptosis/proliferation ratio for patients with high-grade astrocytomas. Neuro-Oncology 4(3):179–186
Tortosa A, Vinolas N, Villa S et al (2003) Prognostic implication of clinical, radiologic, and pathologic features in patients with anaplastic gliomas. Cancer 97(4):1063–1071
Kato H, Fujimura M, Kumabe T, Ishioka C, Kanamaru R, Yoshimoto T (2004) PTEN gene mutation and high MIB-1 labeling index may contribute to dissemination in patients with glioblastoma. J Clin Neurosci 11(1):37–41
Torp SH (1997) Proliferative activity in human glioblastomas: evaluation of different Ki-67 equivalent antibodies. Mol Pathol 50(4):198–200
Chen WJ, He DS, Tang RX, Ren FH, Chen G (2015) Ki-67 is a valuable prognostic factor in gliomas: evidence from a systematic review and meta-analysis. Asian Pac J cancer Prev 16(2):411–420
Vaquero J, Zurita M, Morales C, Oya S, Coca S (2000) Prognostic significance of endothelial surface score and MIB-1 labeling index in glioblastoma. J Neuro-Oncol 46(1):11–16
Persson A, Englund E (2008) Different assessments of immunohistochemically stained Ki-67 and hTERT in glioblastoma multiforme yield variable results: a study with reference to survival prognosis. Clin Neuropathol 27(4):224–233
Dirks P, Bernstein M, Muller PJ, Tucker WS (1993) The value of reoperation for recurrent glioblastoma. Can J Surg J Can de chirurgie 36(3):271–275
Young B, Oldfield EH, Markesbery WR et al (1981) Reoperation for glioblastoma. J Neurosurg 55(6):917–921
Sughrue ME, Sheean T, Bonney PA, Maurer AJ, Teo C (2015) Aggressive repeat surgery for focally recurrent primary glioblastoma: outcomes and theoretical framework. Neurosurg Focus 38(3):E11
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human and animal participants
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Bagley, S.J., Schwab, R.D., Nelson, E. et al. Histopathologic quantification of viable tumor versus treatment effect in surgically resected recurrent glioblastoma. J Neurooncol 141, 421–429 (2019). https://doi.org/10.1007/s11060-018-03050-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-03050-6