Abstract
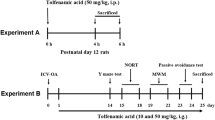
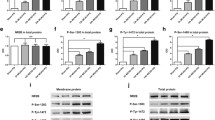
Phosphorylated tau was found to be regulated after cerebral ischemia and linked to high risk for the development of post-stroke dementia. Our previous study showed that ginsenoside Rd (Rd), one of the main active ingredients in Panax ginseng, decreased tau phosphorylation in Alzheimer model. As an extending study, here we investigated whether Rd could reduce tau phosphorylation and sequential cognition impairment after ischemic stroke. Sprague–Dawley rats were subjected to focal cerebral ischemia. The tau phosphorylation of rat brains were analyzed following ischemia by Western blot and animal cognitive functions were examined by Morris water maze and Novel object recognition task. Ischemic insults increased the levels of phosphorylated tau protein at Ser199/202 and PHF-1 sites and caused animal memory deficits. Rd treatment attenuated ischemia-induced enhancement of tau phosphorylation and ameliorated behavior impairment. Furthermore, we revealed that Rd inhibited the activity of Glycogen synthase kinase-3β (GSK-3β), the most important kinase involving tau phosphorylation, but enhanced the activity of protein kinase B (PKB/AKT), a key kinase suppressing GSK-3β activity. Moreover, we found that LY294002, an antagonist for phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathway, abolished the inhibitory effect of Rd on GSK-3β activity and tau phosphorylation. Taken together, our findings provide the first evidence that Rd may reduce cerebral ischemia-induced tau phosphorylation via the PI3K/AKT/GSK-3β pathway.





Similar content being viewed by others
References
Goldstein LB, Adams R, Alberts MJ, Appel LJ, Brass LM, Bushnell CD, Culebras A, DeGraba TJ, Gorelick PB, Guyton JR, Hart RG, Howard G, Kelly-Hayes M, Nixon JV, Sacco RL (2006) Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 113(24):e873–e923. doi:10.1161/01.STR.0000223048.70103.F1
Murray CJ, Lopez AD (1997) Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet 349(9063):1436–1442. doi:10.1016/S0140-6736(96)07495-8
Leys D, Henon H, Mackowiak-Cordoliani MA, Pasquier F (2005) Poststroke dementia. Lancet Neurol 4(11):752–759. doi:10.1016/S1474-4422(05)70221-0
Liu R, Yuan H, Yuan F, Yang SH (2012) Neuroprotection targeting ischemic penumbra and beyond for the treatment of ischemic stroke. Neurol Res 34(4):331–337. doi:10.1179/1743132812Y.0000000020
Tatemichi TK, Paik M, Bagiella E, Desmond DW, Stern Y, Sano M, Hauser WA, Mayeux R (1994) Risk of dementia after stroke in a hospitalized cohort: results of a longitudinal study. Neurology 44(10):1885–1891
de la Torre JC (2004) Is Alzheimer’s disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol 3(3):184–190. doi:10.1016/S1474-4422(04)00683-0
White L, Petrovitch H, Hardman J, Nelson J, Davis DG, Ross GW, Masaki K, Launer L, Markesbery WR (2002) Cerebrovascular pathology and dementia in autopsied Honolulu–Asia Aging Study participants. Ann N Y Acad Sci 977:9–23
Zhang Q, Gao T, Luo Y, Chen X, Gao G, Gao X, Zhou Y, Dai J (2012) Transient focal cerebral ischemia/reperfusion induces early and chronic axonal changes in rats: its importance for the risk of Alzheimer’s disease. PLoS ONE 7(3):e33722. doi:10.1371/journal.pone.0033722
Iwata N, Higuchi M, Saido TC (2005) Metabolism of amyloid-beta peptide and Alzheimer’s disease. Pharmacol Ther 108(2):129–148. doi:10.1016/j.pharmthera.2005.03.010
Kanemaru K (2013) Immunotherapy targeting misfolded proteins in neurodegenerative disease. Brain Nerve 65(4):469–474
Cleveland DW, Hwo SY, Kirschner MW (1977) Physical and chemical properties of purified tau factor and the role of tau in microtubule assembly. J Mol Biol 116(2):227–247
Billingsley ML, Kincaid RL (1997) Regulated phosphorylation and dephosphorylation of tau protein: effects on microtubule interaction, intracellular trafficking and neurodegeneration. Biochem J 323(Pt 3):577–591
Noble W, Olm V, Takata K, Casey E, Mary O, Meyerson J, Gaynor K, LaFrancois J, Wang L, Kondo T, Davies P, Burns M, Veeranna NR, Dickson D, Matsuoka Y, Ahlijanian M, Lau LF, Duff K (2003) Cdk5 is a key factor in tau aggregation and tangle formation in vivo. Neuron 38(4):555–565
Morioka M, Kawano T, Yano S, Kai Y, Tsuiki H, Yoshinaga Y, Matsumoto J, Maeda T, Hamada J, Yamamoto H, Fukunaga K, Kuratsu J (2006) Hyperphosphorylation at serine 199/202 of tau factor in the gerbil hippocampus after transient forebrain ischemia. Biochem Biophys Res Commun 347(1):273–278. doi:10.1016/j.bbrc.2006.06.096
Reynolds CH, Betts JC, Blackstock WP, Nebreda AR, Anderton BH (2000) Phosphorylation sites on tau identified by nanoelectrospray mass spectrometry: differences in vitro between the mitogen-activated protein kinases ERK2, c-Jun N-terminal kinase and P38, and glycogen synthase kinase-3beta. J Neurochem 74(4):1587–1595
Engel T, Hernandez F, Avila J, Lucas JJ (2006) Full reversal of Alzheimer’s disease-like phenotype in a mouse model with conditional overexpression of glycogen synthase kinase-3. J Neurosci 26(19):5083–5090. doi:10.1523/JNEUROSCI.0604-06.2006
Zhu LQ, Wang SH, Liu D, Yin YY, Tian Q, Wang XC, Wang Q, Chen JG, Wang JZ (2007) Activation of glycogen synthase kinase-3 inhibits long-term potentiation with synapse-associated impairments. J Neurosci 27(45):12211–12220. doi:10.1523/JNEUROSCI.3321-07.2007
Cancino GI, Toledo EM, Leal NR, Hernandez DE, Yevenes LF, Inestrosa NC, Alvarez AR (2008) STI571 prevents apoptosis, tau phosphorylation and behavioural impairments induced by Alzheimer’s beta-amyloid deposits. Brain 131(Pt 9):2425–2442. doi:10.1093/brain/awn125
Radad K, Gille G, Liu L, Rausch WD (2006) Use of ginseng in medicine with emphasis on neurodegenerative disorders. J Pharmacol Sci 100(3):175–186
Liu X, Xia J, Wang L, Song Y, Yang J, Yan Y, Ren H, Zhao G (2009) Efficacy and safety of ginsenoside-Rd for acute ischaemic stroke: a randomized, double-blind, placebo-controlled, phase II multicenter trial. Eur J Neurol 16(5):569–575. doi:10.1111/j.1468-1331.2009.02534.x
Liu X, Wang L, Wen A, Yang J, Yan Y, Song Y, Ren H, Wu Y, Li Z, Chen W, Xu Y, Li L, Xia J, Zhao G (2012) Ginsenoside-Rd improves outcome of acute ischaemic stroke—a randomized, double-blind, placebo-controlled, multicenter trial. Eur J Neurol 19(6):855–863. doi:10.1111/j.1468-1331.2011.03634.x
Li XY, Liang J, Tang YB, Zhou JG, Guan YY (2010) Ginsenoside Rd prevents glutamate-induced apoptosis in rat cortical neurons. Clin Exp Pharmacol Physiol 37(2):199–204. doi:10.1111/j.1440-1681.2009.05286.x
Ye R, Li N, Han J, Kong X, Cao R, Rao Z, Zhao G (2009) Neuroprotective effects of ginsenoside Rd against oxygen-glucose deprivation in cultured hippocampal neurons. Neurosci Res 64(3):306–310. doi:10.1016/j.neures.2009.03.016
Ye R, Yang Q, Kong X, Han J, Zhang X, Zhang Y, Li P, Liu J, Shi M, Xiong L, Zhao G (2011) Ginsenoside Rd attenuates early oxidative damage and sequential inflammatory response after transient focal ischemia in rats. Neurochem Int 58(3):391–398. doi:10.1016/j.neuint.2010.12.015
Ye R, Zhang X, Kong X, Han J, Yang Q, Zhang Y, Chen Y, Li P, Liu J, Shi M, Xiong L, Zhao G (2011) Ginsenoside Rd attenuates mitochondrial dysfunction and sequential apoptosis after transient focal ischemia. Neuroscience 178:169–180. doi:10.1016/j.neuroscience.2011.01.007
Ye R, Kong X, Yang Q, Zhang Y, Han J, Li P, Xiong L, Zhao G (2011) Ginsenoside rd in experimental stroke: superior neuroprotective efficacy with a wide therapeutic window. Neurotherapeutics 8(3):515–525. doi:10.1007/s13311-011-0051-3
Ye R, Zhao G, Liu X (2013) Ginsenoside Rd for acute ischemic stroke: translating from bench to bedside. Expert Rev Neurother 13(6):603–613. doi:10.1586/ern.13.51
Li L, Liu J, Yan X, Qin K, Shi M, Lin T, Zhu Y, Kang T, Zhao G (2011) Protective effects of ginsenoside Rd against okadaic acid-induced neurotoxicity in vivo and in vitro. J Ethnopharmacol 138(1):135–141. doi:10.1016/j.jep.2011.08.068
Li L, Liu Z, Liu J, Tai X, Hu X, Liu X, Wu Z, Zhang G, Shi M, Zhao G (2013) Ginsenoside Rd attenuates beta-amyloid-induced tau phosphorylation by altering the functional balance of glycogen synthase kinase 3beta and protein phosphatase 2A. Neurobiol Dis 54:320–328. doi:10.1016/j.nbd.2013.01.002
Liu J, Yan X, Li L, Zhu Y, Qin K, Zhou L, Sun D, Zhang X, Ye R, Zhao G (2012) Ginsennoside rd attenuates cognitive dysfunction in a rat model of Alzheimer’s disease. Neurochem Res 37(12):2738–2747. doi:10.1007/s11064-012-0866-2
Bevins RA, Besheer J (2006) Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc 1(3):1306–1311. doi:10.1038/nprot.2006.205
Li N, Kong X, Ye R, Yang Q, Han J, Xiong L (2011) Age-related differences in experimental stroke: possible involvement of mitochondrial dysfunction and oxidative damage. Rejuvenation Res 14(3):261–273. doi:10.1089/rej.2010.1115
Zhang ZH, Xi GM, Li WC, Ling HY, Qu P, Fang XB (2010) Cyclic-AMP response element binding protein and tau are involved in the neuroprotective mechanisms of nerve growth factor during focal cerebral ischemia/reperfusion in rats. J Clin Neurosci 17(3):353–356. doi:10.1016/j.jocn.2009.07.086
Gordon-Krajcer W, Kozniewska E, Lazarewicz JW, Ksiezak-Reding H (2007) Differential changes in phosphorylation of tau at PHF-1 and 12E8 epitopes during brain ischemia and reperfusion in gerbils. Neurochem Res 32(4–5):729–737. doi:10.1007/s11064-006-9199-3
Kumar P, Miller AI, Polverini PJ (2004) p38 MAPK mediates gamma-irradiation-induced endothelial cell apoptosis, and vascular endothelial growth factor protects endothelial cells through the phosphoinositide 3-kinase–Akt–Bcl-2 pathway. J Biol Chem 279(41):43352–43360. doi:10.1074/jbc.M405777200
Liu RL, Xiong QJ, Shu Q, Wu WN, Cheng J, Fu H, Wang F, Chen JG, Hu ZL (2012) Hyperoside protects cortical neurons from oxygen-glucose deprivation-reperfusion induced injury via nitric oxide signal pathway. Brain Res 1469:164–173. doi:10.1016/j.brainres.2012.06.044
Pap M, Cooper GM (1998) Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem 273(32):19929–19932
Gim SA, Sung JH, Shah FA, Kim MO, Koh PO (2013) Ferulic acid regulates the AKT/GSK-3β/CRMP-2 signaling pathway in a middle cerebral artery occlusion animal model. Lab Anim Res 29(2):63–69. doi:10.5625/lar.2013.29.2.63
Zhu L, Fratiglioni L, Guo Z, Aguero-Torres H, Winblad B, Viitanen M (1998) Association of stroke with dementia, cognitive impairment, and functional disability in the very old: a population-based study. Stroke 29(10):2094–2099
Prencipe M, Ferretti C, Casini AR, Santini M, Giubilei F, Culasso F (1997) Stroke, disability, and dementia: results of a population survey. Stroke 28(3):531–536
Shen H, Wu X, Zhu Y, Sun H (2013) Intravenous administration of achyranthes bidentata polypeptides supports recovery from experimental ischemic stroke in vivo. PLoS ONE 8(2):e57055. doi:10.1371/journal.pone.0057055
Zhang X, Yeung PK, McAlonan GM, Chung SS, Chung SK (2013) Transgenic mice over-expressing endothelial endothelin-1 show cognitive deficit with blood–brain barrier breakdown after transient ischemia with long-term reperfusion. Neurobiol Learn Mem 101:46–54. doi:10.1016/j.nlm.2013.01.002
Roof RL, Schielke GP, Ren X, Hall ED (2001) A comparison of long-term functional outcome after 2 middle cerebral artery occlusion models in rats. Stroke 32(11):2648–2657
Tatemichi TK, Foulkes MA, Mohr JP, Hewitt JR, Hier DB, Price TR, Wolf PA (1990) Dementia in stroke survivors in the Stroke Data Bank cohort. Prevalence, incidence, risk factors, and computed tomographic findings. Stroke 21(6):858–866
Dong DW, Zhang YS, Yang WY, Wang-Qin RQ, Xu AD, Ruan YW (2013) Hyperphosphorylation of tau protein in the ipsilateral thalamus after focal cortical infarction in rats. Brain Res. doi:10.1016/j.brainres.2013.11.004
Wada A, Yokoo H, Yanagita T, Kobayashi H (2005) Lithium: potential therapeutics against acute brain injuries and chronic neurodegenerative diseases. J Pharmacol Sci 99(4):307–321
Shi C, Zheng DD, Fang L, Wu F, Kwong WH, Xu J (2012) Ginsenoside Rg1 promotes nonamyloidgenic cleavage of APP via estrogen receptor signaling to MAPK/ERK and PI3K/Akt. Biochim Biophys Acta 1820(4):453–460. doi:10.1016/j.bbagen.2011.12.005
Hwang YP, Jeong HG (2010) Ginsenoside Rb1 protects against 6-hydroxydopamine-induced oxidative stress by increasing heme oxygenase-1 expression through an estrogen receptor-related PI3K/Akt/Nrf2-dependent pathway in human dopaminergic cells. Toxicol Appl Pharmacol 242(1):18–28. doi:10.1016/j.taap.2009.09.009
Zhang X, Shi M, Bjoras M, Wang W, Zhang G, Han J, Liu Z, Zhang Y, Wang B, Chen J, Zhu Y, Xiong L, Zhao G (2013) Ginsenoside Rd promotes glutamate clearance by up-regulating glial glutamate transporter GLT-1 via PI3K/AKT and ERK1/2 pathways. Front Pharmacol 4:152. doi:10.3389/fphar.2013.00152
Acknowledgments
The authors are grateful to Ms. Dongyun Feng for technical assistance. This study was supported by the National Natural Science Foundation of China (Nos. 81171236, 81371365, 31170801, and 31300900) and Program for Changjiang Scholars and Innovative Research Team in University (No. IRT1053).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, X., Shi, M., Ye, R. et al. Ginsenoside Rd Attenuates Tau Protein Phosphorylation Via the PI3K/AKT/GSK-3β Pathway After Transient Forebrain Ischemia. Neurochem Res 39, 1363–1373 (2014). https://doi.org/10.1007/s11064-014-1321-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1321-3