Abstract
Why does life use α-amino acids exclusively as building blocks of proteins? To address that fundamental question from an energetic perspective, this study estimated the standard molal thermodynamic data for three non-α-amino acids (β-alanine, γ-aminobutyric acid, and ε-aminocaproic acid) and α-amino-n-butyric acid in their zwitterionic, negative, and positive ionization states based on the corresponding experimental measurements reported in the literature. Temperature dependences of their heat capacities were described based on the revised Helgeson–Kirkham–Flowers (HKF) equations of state. The obtained dataset was then used to calculate the standard molal Gibbs energies (∆G o) of the non-α-amino acids as a function of temperature and pH. Comparison of their ∆G o values with those of α-amino acids having the same molecular formula showed that the non-α-amino acids have similar ∆G o values to the corresponding α-amino acids in physiologically relevant conditions (neutral pH, <100 °C). In acidic and alkaline pH, the non-α-amino acids are thermodynamically more stable than the corresponding α-ones over a broad temperature range. These results suggest that the energetic cost of synthesis is not an important selection pressure to incorporate α-amino acids into biological systems.
Similar content being viewed by others
Introduction
Why does life use α-amino acids exclusively as building blocks of proteins? To resolve that fundamental question, extensive discussion has progressed from various scientific viewpoints, including (1) the availability of amino acids on the primitive Earth, (2) the stability of amino acids relative to decomposition and racemization, (3) functional utility in biochemical processes, and (4) the energetic cost of synthesis (Weber and Miller 1981; Cleaves 2010). The fourth factor was evaluated previously by Cleaves (2010) through comparison of the enthalpy of formation (∆ f H) of several α-amino acids with β-amino acids containing the same side-chain structures. No meaningful enthalpic difference was observed, implying that the energetic cost is not an important selection pressure to incorporate α-amino acids into biological systems. This result suggests that structural differences between the two are more important factor.
The values of ∆ f H used in that evaluation represent the enthalpy of amino acids at a physiological condition. It is noteworthy, however, that amino acids show vastly different thermodynamic properties depending on the environmental conditions. With increasing pH from acidic to alkaline, for instance, the net charge of an amino acid molecule changes from positive to neutral to negative because of the deprotonation of the functional groups (−NH3 + → −NH2 + H+, −COOH → −COO− + H+). Each ionization state has mutually different thermodynamic properties (Kitadai 2014, 2015). Additionally, their properties have mutually different temperature dependences. Because various environments have been proposed as important sites for the chemical evolution of amino acids on the Hadean Earth (e.g., submarine alkaline hydrothermal systems by Sakata et al. (2010) and acidic tidal pools on beaches by Rode (1999)), evaluating the synthetic costs of non-α-amino acids over broad temperature and pH ranges is worthwhile, as is comparing their values with those of corresponding α-amino acids.
As a starting point to this end, this study determined basic sets of the standard molal thermodynamic data for three non-α-amino acids (β-alanine (β-Ala), γ-aminobutyric acid (γ-ABA), and ε-aminocaproic acid (ε-ACA)) and α-amino-n-butyric acid (α-ABA) in their zwitterionic, negative, and positive ionization states based on the corresponding experimental measurements reported in the literature. Temperature dependences of their heat capacities were described based on the revised Helgeson–Kirkham–Flowers (HKF) equations of state (Helgeson et al. 1981; Tanger and Helgeson 1988). These four amino acids were selected because they have been synthesized in many prebiotic experiments simulating the surface environment of the Hadean Earth or the early solar nebula (Zaia et al. 2008). In fact, these have been detected from almost all types of carbonaceous chondrites (Glavin et al. 2011). β-Ala is among the most abundant amino acids identified in CI carbonaceous chondrites (Glavin et al. 2011; Burton et al. 2014), whereas εACA is in CH and CB chondrites (Burton et al. 2013). γ-ABA and α-ABA typically exhibit intermediate concentrations between the two (Glavin et al. 2011; Burton et al. 2013, 2014). Therefore, the four amino acids could have influenced the selection of α-amino acids for protein synthesis during the origin and early evolution of life. Another significance for the four amino acids is that these have different numbers of carbon atoms between the amino group (−NH3 +) and the carboxyl group (−COO−) (one, α-ABA; two, β-Ala; three, γ-ABA; five, ε-ACA). Comparison of their thermodynamic properties therefore allows evaluation of the contribution from the intramolecular interaction of the two functional groups. That information is expected to be useful to discuss why life exclusively uses amino acids with the shortest -NH3 + ↔ −OOC- distance (i.e., α-amino acids).
Calculation Methodology
The thermodynamic data and revised HKF parameters for α-ABA, β-Ala, γ-ABA, and ε-ACA in their zwitterionic, positive and negative ionization states were obtained in the manner presented below. The thermodynamic conventions and the revised HKF equations of state adopted for this study are explained in Appendix 1.
Standard Molal Thermodynamic Data for non-Protein Amino Acids at 25 °C and 1 bar
Table 1 presents values of the standard molal Gibbs energy (∆ f G°), enthalpy (∆ f H°) and entropy (S°) at 25 °C and 1 bar for the four non-protein amino acids. The values of ∆ f G° for zwitterionic β-Ala and γ-ABA (β-Ala± and γ-ABA±, respectively) were calculated from ∆ f G° of the respective crystalline states (Δ f G o cr ) by consideration of their solubilities in water at 25 °C and 1 bar (M sat = 7.869 mol kg−1 for β-Ala± and 11.25 mol kg−1 for γ-ABA±; Romero and Oviedo 2013) together with their activity coefficients (γ sat ).
In this equation, T and R respectively stand for the temperature in Kelvin and the gas constant (1.98721 cal mol−1 K−1). The γ sat of the zwitterionic species were referred from a report by Lilley (1985), where the values in near-saturated aqueous solutions of the respective amino acids are presented. The Δ f G o cr of crystalline β-Ala± and γ-ABA± was calculated using ∆ f H° of the respective solids at 25 °C and 1 bar (Δ f H o cr =–133.63 kcal mol– 1 for β-Ala±; da Silva et al. 2010 and −138.87 kcal mol−1 for γ-ABA±; Skoulika and Sabbah 1983) together with those of S° at 25 °C and 1 bar (\( {S}_{Cr,\ {P}_r,{T}_r}^o=30.26\ \mathrm{cal}\ {\mathrm{mol}}^{\hbox{--} 1}{\mathrm{K}}^{\hbox{--} 1} \) for β-Ala±, Paukov et al. 2009; and 37.89 cal mol−1 K−1 for γ-ABA±, Paukov et al. 2013) as shown below.
Here, \( {S}_{P_r,\ {T}_r,\ elements}^o \) represents the total standard molal entropy at 25 °C and 1 bar of the elements making up the species of interest (O2(g), H2(g), C(graphite), and N2(g)). The values of S° for the elements were taken from a report by Cox et al. (1989).
The ∆ f H° of aqueous β-Ala± and γ-ABA± were calculated using the Δ f H o cr of respective solids described above by consideration of their standard enthalpies of solution at 25 °C and 1 bar (∆ sol H o = 1.91 kcal mol−1 for β-Ala± and −0.15 kcal mol−1 for γ-ABA±, Prasad and Ahluwalia 1976) as presented below.
The values of S° at 25 °C and 1 bar for aqueous β-Ala± and γ-ABA± were subsequently calculated from Eq. 2 using the values of ∆ f G o and ∆ f H o presented in Table 1.
The values of ∆ f G°, ∆ f H°, and S° at 25 °C and 1 bar for α-ABA± were reported previously by Shock and Helgeson (1990) (−87.12 kcal mol−1, −138.18 kcal mol−1, and 46.7 cal mol−1 K−1, respectively). However, because of the paucity of experimental data for α-ABA± at that time, these values were estimated provisionally using the correlation between the standard molal properties of α-amino acids and the number of carbon atoms in their structures (e.g., ∆ f H° of α-ABA± = −5.67 × 4–115.50 kcal mol−1). More recently, the Δ f H o cr of crystalline α-ABA± was determined experimentally as −138.77 kcal mol−1 (Yang et al. 1999). That value, together with the ∆ sol H o of α-ABA± reported by Prasad and Ahluwalia (1976) (1.58 kcal mol−1), gives the ∆ f H° of aqueous α-ABA± = −137.19 kcal mol−1 (calculated from Eq. 3). This ∆ f H° value was used for this study. It was used in combination with the S° at 25 °C and 1 bar as estimated by Shock and Helgeson (1990), to calculate the ∆ f G° for α-ABA± (−86.13 kcal mol−1, calculated from Eq. 2).
The values of ∆ f G°, ∆ f H° and S° at 25 °C and 1 bar for ε-ACA± were obtained as follows. First, ∆ f H° was calculated from Eq. 3 using the reported Δ f H o cr (−153.35 kcal mol−1, Contineanu et al. 2005) and ∆ sol H o (−0.33 kcal mol−1, Prasad and Ahluwalia 1976) of crystalline ε-ACA±. Second, the provisional estimate of S° at 25 °C and 1 bar was calculated using group additivity algorithms developed by Amend and Helgeson (1997; 2000) and Dick et al. (2006). Based on that algorithm, the standard molal thermodynamic data and the revised HKF parameters for ε-ACA± (\( {\varXi}_{\varepsilon - AC{A}^{\pm }} \)) are calculable using the corresponding values of γ-ABA± (\( {\Xi}_{\gamma - AB{A}^{\pm }} \)) together with those of the [−CH2-] group (\( {\Xi}_{\left[-C{H}_2\right]} \)) as shown below.
The \( {\Xi}_{\gamma - AB{A}^{\pm }} \) used in this calculation was taken from Table 1. Also, \( {\Xi}_{\left[-C{H}_2\right]} \) was referred from a report by Dick et al. (2006). The uncertainty of S° at 25 °C and 1 bar associated with the additivity prediction is ±0.50 cal mol−1 K−1 (Amend and Helgeson 1997; Dick et al. 2006). The value of S° was then used together with the values of ∆ f H° obtained using the procedure described above, to calculate the ∆ f G° of ε-ACA± from Eq. 2 (−85.78 kcal mol−1).
The values of ∆ f G o, ∆ f H o, and S° at 25 °C and 1 bar for the ionization states of the non-protein amino acids were calculated from the values of the corresponding zwitterionic states (Table 1) in combination with the standard molal properties of ionization (∆ i G o, ∆ i H o, and ∆ i S o) reported in the literature (Price et al. (2003) for α-ABA+ and α-ABA−, Smith and Martell (2004) for β-Ala+ and β-Ala−, King (1954) for γ-ABA+ and γ-ABA−, Christensen et al. (1968) for ε-ACA+, and Smith and Smith (1942) for ε-ACA−) in accord with the following.
and
Retrieval of the Revised HKF Equations of State Parameters for Heat Capacity
The values of the standard molal heat capacity (C o P ) at 25 °C and 1 bar and the revised HKF parameters for the temperature dependence of C o P (c 1 , c 2 and ω; see Appendix 1) of the four non-protein amino acids were obtained as described below.
All values for β-Ala± were referred from Plyasunov and Shock (2001), which allows prediction of the temperature dependence of C o P of β-Ala± consistent with experimentally obtained results (Gucker and Allen 1942; Clarke et al. 2000; Hakin and Liu 2006; Fig. 1a). The values for α-ABA± were determined previously by Shock and Helgeson (1990). However, because of the paucity of experimental data for α-ABA± at that time, these values were estimated provisionally with the aid of a correlation between the values of C o P at 25 °C and 1 bar and c 2 for several carboxylic acids (HCOOH, CH3COOH, and C2H5COOH). Consequently, the estimated values do not accurately represent the temperature dependence of C o P for α-ABA± reported in the literature (Hakin et al. 1994; Price et al. 2003; Fig. 1b). The c 1 , c 2 , and ω values of α-ABA± were therefore updated for the present study by simultaneous regression of experimental C o P data as a function of temperature (Hakin et al. 1994; Price et al. 2003) with Eq. 14. Figure 2 shows the regression as a line on plots of C o P vs. 1/(T–Θ)2. A value of ω was chosen to linearize the trend of data points in the regression plots in this figure based on least-squares method. It follows from Eq. 14 that the intercept and slope of the regression line respectively correspond to c 1 and c 2 . The obtained values were then used to calculate the C o P of α-ABA± at 25 °C and 1 bar from Eq. 14. The temperature dependence of C o P predicted for α-ABA± calculated using the parameters presented in Table 1 is closely consistent with the experimental data (Fig. 1b). The same methodology was applied for retrieval of the c 1 , c 2 , ω, and C o P values for α-ABA+, α-ABA−, β-Ala+, β-Ala−, γ-ABA±, γ-ABA+ and γ-ABA− (Figs. 1a,b and c, and Table 1).
Experimental (symbols) and calculated (curves) standard molal heat capacity (C o P ) of a β-alanine, b α-aminobutyric acid, c γ-amino-n-butyric acid, and d ε-aminocaproic acid in their zwitterionic, positive and negative ionization states as a function of temperature. No error bar are shown in this figure because the reported uncertainty associated with experimental data is less than the symbol size
The methodology is not applicable for ε-ACA± because of the paucity of experimental C o P data as a function of temperature. The values of c 2 and ω for ε-ACA± were therefore estimated provisionally from Eq. 4 using the corresponding values of γ-ABA± (Table 1) and those of [−CH2-] reported by Dick et al. (2006). The c 1 for ε-ACA± was then computed from Eq. 14 using the estimated c 2 and ω values together with the experimental C o P data at 25 °C and 1 bar (278.0 J mol−1 K−1; Ahluwalia et al. 1977). The values of c 1 , c 2 , and ω and C o P at 25 °C and 1 bar for ε-ACA+ and ε-ACA− were subsequently obtained by (1) computing the temperature dependence of C o P of ε-ACA± from Eq. 14 (Fig. 1d), by (2) calculating the C o P of ε-ACA+ and ε-ACA− as a function of temperature using that of ε-ACA± (Fig. 1d) in combination with the heat capacities of protonation of ε-ACA± and ε-ACA− reported in the literature by Christensen et al. (1968) and Wang et al. (1996) for ε-ACA± + H+ → ε-ACA+, and by Smith and Smith (1942) and Gillespie et al. (1995) for (ε-ACA− + H+ → ε-ACA±), and by (3) regressing the data to retrieve the c 1 , c 2 , and ω and C o P for ε-ACA + and ε-ACA− using the procedure described above.
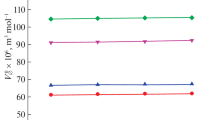
Table 1 also presents values of the standard molal volumes (V o) at 25 °C and 1 bar for non-protein amino acids reported in the literature (Price et al. (2003) for α-ABA±, α-ABA+, and α-ABA−, Shahidi and Farrell (1978) for β-Ala±, γ-ABA± and ε-ACA±, Shahidi (1980) for β-Ala+, β-Ala−, γ-ABA+, γ-ABA−, ε-ACA+, and ε-ACA−).
Computational Uncertainties
Uncertainties associated with the thermodynamic data and revised HKF parameters for the four non-protein amino acids (defined here as Ξ unc. ) are presented in Table 2. The values of Δ f G o unc. , Δ f H o unc. , and S o unc. at 25 °C and 1 bar for α-ABA±, α-ABA+, α-ABA−, β-Ala±, β-Ala+, β-Ala−, γ-ABA±, γ-ABA+ and γ-ABA− were assessed from the reported experimental uncertainties. The value of S o unc. for ε-ACA± was estimated to be ±0.50 cal mol−1 K−1 (Amend and Helgeson 1997; Dick et al. 2006). The value was used, together with the Δ f H o unc. for ε-ACA± (assessed to be ±0.30 kcal mol−1 from the uncertainties in the Δ f H o cr (0.28 kcal mol−1, Contineanu et al. 2005) and the ∆ sol H o (0.02 kcal mol−1, Prasad and Ahluwalia 1976) of crystalline ε-ACA±), to calculate the Δ f G o unc. for ε-ACA± to be ±0.45 kcal mol−1. The uncertainties in ∆ f G°, ∆ f H°, and S° for ε-ACA+ and ε-ACA− were then calculated from the corresponding uncertainties for ε-ACA± in combination with those for the ionization of ε-ACA± reported in the literature (Smith and Smith 1942; Christensen et al. 1968).
The uncertainties in C o P , c 1 , c 2 and ω for α-ABA±, α-ABA+, α-ABA−, β-Ala+, β-Ala−, γ-ABA±, γ-ABA+ and γ-ABA− were estimated from the scatter of the experimental data points in the regression calculations (Fig. 2). Those for β-Ala± and ε-ACA± were referred from Shock and Helgeson (1990) and Amend and Helgeson (1997). The values of C o p, unc. , c 1,unc. , c 2,unc. , and ω unc. for ε-ACA+ and ε-ACA− were calculated by combining the corresponding uncertainties for ε-ACA± with those arose from the regression calculations for their C o P values as a function of temperature.
Table 2 also presents the reported experimental uncertainties in V o at 25 °C and 1 bar for the four non-protein amino acids.
The uncertainties estimated above (Table 2) can be used to calculate temperature dependences of the Δ f G o unc. . Fig. 3 shows the values of Δ f G o unc. as a function of temperature (0–200 °C) for the four non-protein amino acids calculated by combining the contributions from the uncertainty in the data and parameters for the respective amino acids presented in Table 2 using Eq. 16. α-ABA±, α-ABA+ and α-ABA− showed relatively large values of Δ f G o unc. over the examined temperature range, whereas those for β-Ala±, ε-ACA±, ε-ACA+ and ε-ACA− increased rapidly at >50 °C (Fig. 3). The former uncertainties originate majorly from the uncertainty in the Δ f H o cr of crystalline α-ABA± (±1.22 kcal mol−1; Yang et al. 1999), whereas the latter from the uncertainties in ω (~2.5 cal mol−1; Table 2). Note that the Δ f G o unc. values correspond to maximum uncertainties because the additive contributions from each data and parameter may either increase or decrease the value of Δ f G o unc. . Cancellation of these contributions would result in lower uncertainties than those shown in this figure.
Uncertainties in the value of ∆fG° (Δ f G o unc. ) for the four non-protein amino acids as a function of temperature calculated using the uncertainties in the data and parameters for the respective amino acids presented in Table A1 from Eq. 16
Thermodynamic Behaviors of Non-α-Amino Acids as a Function of Temperature and pH
The standard molal thermodynamic data and the revised HKF parameters obtained in this study are useful for predicting the thermodynamic behaviors of non-protein amino acids over wide ranges of temperature and pH. For instance, Fig. 4 shows the protonation constants (logK prot. ) of α-ABA (Fig. 4b), β-Ala (Fig. 4c), γ-ABA (Fig. 4d), and ε-ACA (Fig. 4e), together with the corresponding experimental measurements reported in the literature, for temperatures of 0–200 °C. For comparison, the logK prot. values for Ala (Kitadai 2015) are also depicted in Fig. 4a. The curves were calculated by the following equation.
Therein, ∆ r G o denotes the standard Gibbs energy of reaction, as calculated using combining the standard molal Gibbs energies (∆G o) of the individual compounds involved in the reaction.
The ∆G o of the non-protein amino acids at any temperature was calculated from Eqs. 13 and 14 using the data and parameters presented in Table 1. Pressure was set as 1 bar at <100 °C, with saturated water vapor pressure (P sat ) at ≥100 °C. It is apparent from Fig. 4 that the predicted curves show close agreement with the experimental measurements for each reaction at all temperatures. Experimental logK prot. data at >100 °C were obtained under various pressures ranging from the saturated water vapor pressure (P sat ) (Hamborg et al. 2007) up to 1.52 MPa (Wang et al. 1996). However, the influence of pressure on the protonation ∆ r G o of amino acid is small (e.g., an increase of pressure from P sat to 250 bar is expected to increase the protonation ∆ r G o of Ala− by 0.01 kcal mol−1 at 100 °C and by −0.02 kcal mol−1 at 200 °C (Kitadai 2015)). Consequently, the agreement between the experimental (symbols) and calculated (curves) logK prot. values (Fig. 4) supports the consistency of the dataset presented in Table 1.
The values of logK prot. for the zwitterionic state (AA± + H+ → AA+) and the anionic state (AA− + H+ → AA±) increased concomitantly with increasing distance between the amino group and the carboxyl group in an amino acid structure (\( {D}_{N{H}_3^{+}\leftrightarrow {\mathrm{COO}}^{-}} \)) in all the examined temperature range (Fig. 4). The positive correlation is explainable by consideration of the entropic (Δ r S o prot. ) and enthalpic (Δ r H o prot. ) contributions to the protonation Gibbs energy (Δ r G o prot. ):
As might be apparent from Table 3, the values of TΔ r S o prot. for the zwitterionic amino acids increased concomitantly with increasing the \( {D}_{N{H}_3^{+}\leftrightarrow {\mathrm{COO}}^{-}} \), whereas those of Δ r H o prot. showed no clear correlation. The values of Δ r S o prot. are influenced primarily by the reduction in the number of solute species (e.g., β-Ala± + H+ → β-Ala+; two → one) and by the release of H2O molecules from the hydration spheres of charged species when an amino acid is protonated (Gillespie et al. 1995). The reduction in the number of solute species is the same among the amino acids examined. Therefore, the difference in the TΔ r S o prot. value is attributable to the release of H2O molecules. The H2O molecules in the hydration spheres are in a more ordered state than those in bulk water because of the interaction between the water dipoles and the charged moieties. Consequently, the entropy of the bulk system increases when protonation occurs because H2O molecules influenced by H+ and the carboxyl group (−COO−) are released to a higher entropy state of H2O molecules in the bulk water. The increase is smaller in the case of α-amino acids (Ala± and α-ABA±) because the proximity of the amino group (−NH3 +) and the carboxyl group (−COO−) partially neutralizes their charges in their structure, resulting in less interaction with H2O molecules. For ε-ACA±, the separated zwitterion charges influence an increasing number of water molecules. Consequently, more water molecules are released to the bulk phase, resulting in a higher TΔ r S o prot. value.
A similar explanation is applicable for the decrease in the TΔ r S o prot. of the anionic amino acids with increasing the \( {D}_{N{H}_3^{+}\leftrightarrow {\mathrm{COO}}^{-}} \) (Table 3). Aside from the TΔ r S o prot. , the Δ r H o prot. of the anionic amino acids showed negative correlation with \( {D}_{N{H}_3^{+}\leftrightarrow {\mathrm{COO}}^{-}} \) (Table 3). The values of Δ r H o prot. are all negative (i.e., exothermic reaction) because, through the protonation, an electric energy sufficient to separate the negatively charged amino acid (AA−) and positively charged H+ ion is released into the bulk system. The released energy increases concomitantly with increasing \( {D}_{N{H}_3^{+}\leftrightarrow {\mathrm{COO}}^{-}} \) because the charge separation results in higher stabilization of the zwitterionic amino acids as a result of the stronger -NH3 + ↔ H2O interaction. The increase of the \( {D}_{N{H}_3^{+}\leftrightarrow {\mathrm{COO}}^{-}} \) causes a steeper decrease of the Δ r H o prot. than the increase of the TΔ r S o prot. (Table 3); so the logK prot. values increased in the order of Ala ≈ α-ABA < β-Ala < γ-ABA < ε-ACA.
How do the different protonation behaviors arising from the distance of -NH3 + ↔ –OOC- influence the Gibbs energy of formation of amino acids? To examine the influence, the ∆G o of β-Ala, γ-ABA, and ε-ACA were calculated as a function of temperature and pH, and were compared with those of α-amino acids having the same molecular formula (β-Ala vs. Ala, γ-ABA vs. α-ABA, and ε-ACA vs. Leu; Fig. 5). At neutral pH, the non-α-amino acids showed similar ∆G o values with the corresponding α-amino acids over the entire examined temperature range (25–200 °C) (i.e., the differences in ∆G o between the non-α-amino acids and α-amino acids close to the error; Fig. 3). In contrast, negative ∆G o values were calculated for the differences at acidic and alkaline pH, except for ε-ACA at the highest temperature (200 °C). In the two pH regions, amino acids change their ionization states from negative to zwitterionic (alkaline pH → neutral pH) and from zwitterionic to positive (neutral pH → acidic pH). Ionization states of amino acids have mutually different ∆G o values with the magnitude decreasing in the order of AA− > AA± > AA+ (Table 1). With decreasing pH from alkaline to acidic, the ∆G o of amino acids decreases because of the change in the ionization state from negative to zwitterionic to positive. Because the non-α-amino acids have higher logK prot. values than the α-amino acids have (Fig. 4), the decrease in ∆G o occurs at higher pH. Consequently, the non-α-amino acids show smaller ∆G o values in the pH region between the logK prot of the non-α-amino acids and the logK prot of the α-amino acids.
Differences in the standard molal Gibbs energy of formation between a β-Ala and Ala, b γ-ABA and α-ABA, and c ε-ACA and Leu as a function of pH at 25, 100, 150, and 200 °C, calculated using the thermodynamic data and the revised HKF parameters presented in Table 1 for the non-protein amino acids and those reported by Kitadai (2015) for the protein amino acids. The ionic strength was set to 0.1 (NaCl). The activity coefficients for aqueous species were calculated using the extended Debye–Hückel equation (Helgeson et al. 1981). Dashed vertical lines represent neutral pH positions (the pH where the concentration of H+ equals to that of OH−) at respective temperatures
The calculation for ∆G o (Fig. 5) cannot clearly explain life’s exclusive usage of α-amino acids as building blocks of proteins because α-amino acids showed no meaningful difference in the ∆G o value from the corresponding non-α-amino acids in physiologically relevant conditions (neutral pH, <100 °C). In acidic and alkaline pH, α-amino acids are thermodynamically less stable than the corresponding non-α-ones over a broad temperature range (Fig. 5). However, selection of α-amino acids is still possible even in the two pH regions if α-amino acids have important benefits in biochemical utility or in other physicochemical properties sufficient to overcome the thermodynamic disadvantage (Weber and Miller 1981; Cleaves 2010). A possible advantage of α-amino acids over non-α-ones is their tendency to form more stable structures in protein synthesis (Weber and Miller 1981). Insertion of carbon atoms between the amino and carboxyl groups leads to greater conformational freedom caused by internal rotations about the C-C bond, which makes it difficult to form ordered secondary structures.
It is also conceivable that life emerged from environments rich in energy and nutrients where the synthesis of α-amino acids (and non-α-amino acids) is thermodynamically favorable. Promising candidates proposed so far are Hadean alkaline hydrothermal settings, where fluid mixing between hot and alkaline hydrothermal fluids (>100 °C, pH ≥9) and cool and slightly acidic Hadean ocean water (≤25 °C, pH 5–6) generates energetically suitable conditions for the synthesis of α-amino acids (Amend and McCollom 2009; Kitadai 2015). The ∆G o of α-amino acid synthesis from simple inorganic precursors (e.g., CO2 and NH3) depend greatly on the mixing ratio of the hydrothermal fluid and seawater as well as the compositions of the two end-member fluids (Amend and McCollom 2009; Kitadai 2015). In such conditions, the amino acid composition may be controlled by kinetics rather than thermodynamics; so preferential formations of α-amino acids may be possible in the presence of effective inorganic catalysts. Wachtershauser and co-workers have shown that a hydrothermal heating of CO and CN− in the presence of Fe/Ni precipitates (sulfides or hydroxides) effectively generate α-amino acids including glycine, alanine, and serine (Huber and Wachtershauser 2006; Huber et al. 2012). Non-α-amino acid has not been observed in their experiments. Conversely, if life emerged in energetically limited environments, then selection pressure for less costly amino acids is expected to be a non-negligible factor. Consequently, the usage of non-α-amino acids might present an alternative.
Concluding Remarks
This study estimated the standard molal thermodynamic data at 25 °C and 1 bar and the revised HKF parameters for the temperature dependence of C o P for four non-protein amino acids (α-ABA, β-Ala, γ-ABA, and ε-ACA). Thermodynamic calculations using the dataset showed that non-α-amino acids are thermodynamically more stable than α-amino acids with the same molecular formula in acidic and alkaline pH regions over a broad temperature range. This result suggests that the energetic cost is not an important selection pressure to incorporate α-amino acids into biological systems. It is noteworthy that a discrepancy exists between simple thermodynamic calculations and actual energetic costs for amino acid synthesis in metabolic processes (Akashi and Gojobori 2002; Higgs and Pudritz 2009). Consequently, the dataset obtained in this study alone cannot lead to a comprehensive understanding of the role of energetics in the selection of α-amino acids. The point of this study is to provide a quantitative base for future investigation of this topic. I also recognize that many non-protein amino acids (e.g., isovaline) can be regarded as competitors of α-amino acids besides the four considered here. Determination of the thermodynamic properties of these amino acids must be done later, at time when sufficient experimental data are available.
References
Ahluwalia JC, Ostiguy C, Perron G, Desnoyers JE (1977) Volumes and heat capacities of some amino acids in water at 25 °C. Can J Chem 55:3364–3367
Akashi H, Gojobori T (2002) Metabolic efficiency and amino acid composition in the proteomes of Escherichia coli and Bacillus subtilis. Proc Natl Acad Sci U S A 99:3695–3700
Amend JP, Helgeson HC (1997) Calculation of the standard molal thermodynamic properties of aqueous biomolecules at elevated temperatures and pressures. Part 1. L-α-amino acids. J Chem Soc Faraday Trans 93:1927–1941
Amend JP, Helgeson HC (2000) Calculation of the standard molal thermodynamic properties of aqueous biomolecules at elevated temperatures and pressures. II. Unfolded proteins. Biophys Chem 84:105–136
Amend JP, McCollom TM (2009) Energetics of biomolecule synthesis on early earth. In: Zaikowski L, Freidrich JM, Seidel SR (eds) Chemical evolution II: from the origins of life to modern society. American chemical society symposium series. Oxford University Press, New York, pp 6–94
Burton AS, Elsila JE, Hein JE, Glavin DP, Dworkin JP (2013) Extraterrestrial amino acids identified in metal-rich CH and CB carbonaceous chondrites from Antarctica. Meteorit Planet Sci 48:390–402
Burton AS, Grunsfeld S, Elsila JE, Glavin DP, Dworkin JP (2014) The effects of parent-body hydrothermal heating on amino acid abundances in CI-like chondrites. Polar Sci 8:255–263
Christensen JJ, Oscarson JL, Izatt RM (1968) Thermodynamics of proton ionization in dilute aqueous solution. X. ∆G° (pK), ∆H°, and ∆S° values for proton ionization from several monosubstituted carboxylic acids at 10, 25, 40°. J Am Chem Soc 90:5949–5953
Clarke RG, Hnedkovsky L, Tremaine PR, Majer V (2000) Amino acids under hydrothermal conditions: apparent heat capacities of aqueous α-alanine, β-alanine, glycine, and proline at temperatures from 298 to 500 K and pressures up to 30.0 MPa. J Phys Chem B 104:11781–11793
Cleaves HJ (2010) The origin of the biologically coded amino acids. J Theor Biol 263:490–498
Contineanu I, Chivu L, Perisanu S (2005) The enthalpies of combustion and formation of L-α-glutamic and 6-aminohexanoic acids. J Therm Anal Calorim 82:3–6
Cox JD, Wagman DD, Medvedev VA (1989) COVATA key values for thermodynamics. Hemisphere Publishing Corp, New York
da Silva MAVR, da Silva MDMCR, Santos AFLOM, Roux MV, Foces-Foces C, Notario R, Guzman-Mejia R, Juaristi E (2010) Experimental and computational thermodynamical study of α-alanine (DL) and β-alanine. J Phys Chem B 114:16471–16480
Dick JM, LaRowe DE, Helgeson HC (2006) Temperature, pressure, and electrochemical constraints on protein speciation: group additivity calculation of the standard molal thermodynamic properties of ionized unfolded proteins. Biogeosciences 3:311–336
Gillespie SE, Oscarson JL, Izatt RM, Wang P, Renuncio JAR, Pando C (1995) Thermodynamic quantities for the protonation of amino acid amino groups from 323.15 to 398.15 K. J Solut Chem 24:1219–1247
Glavin DP, Callahan MP, Dworkin JP, Elsila JE (2011) The effects of parent body processes on amino acids in carbonaceous chondrites. Meteorit Planet Sci 45:1948–1972
Gucker FT, Allen TW (1942) The densities and specific heats of aqueous solutions of dl-α-alanine, β-alanine and lactamide. J Am Chem Soc 64:191–199
Hakin AW, Liu JL (2006) The calorimetric and volumetric properties of selected α-amino acids and α, ω-amino acids in water at T = (288.15, 298.15, 313.15, and 328.15) K and p = 0.1 MPa. J Solut Chem 35:1157–1171
Hakin AW, Duke MM, Marty JL, Preuss KE (1994) Some thermodynamic properties of aqueous amino acid systems at 288.15, 298.15, 313.15 and 328.15 K: group additivity analyses of standard-state volumes and heat capacities. J Chem Soc Faraday Trans 90:2027–2035
Hamborg ES, Niederer JPM, Versteeg GF (2007) Dissociation constants and thermodynamic properties of amino acids used in CO2 absorption (from 293 to 353) K. J Chem Eng Data 52:2491–2502
Helgeson HC, Kirkham DH, Flowers GC (1981) Theoretical prediction of the thermodynamic behavior of aqueous electrolytes at high pressures and temperatures: IV. Calculation of activity coefficients, osmotic coefficients, and apparent molal and standard and relative partial molal properties to 600 °C and 5 kb. Am J Sci 281:1249–1516
Higgs PG, Pudritz RE (2009) A thermodynamic basis for prebiotic amino acid synthesis and the characters of the first genetic code. Astrobiology 9:483–490
Huber C, Wachtershauser G (2006) α-hydroxy and α-amino acids under possible Hadean, volcanic origin-of-life conditions. Science 314:630–632
Huber C, Kraus F, Hanzlik M, Eisenreich W, Wachtershauser G (2012) Elements of metabolic evolution. Chem Eur J 18:2063–2080
King EJ (1954) The thermodynamics of ionization of amino acids. I The ionization constants of γ-aminobutyric acid. J Am Chem Soc 76:1006–1008
Kitadai N (2014) Thermodynamic prediction of glycine polymerization as a function of temperature and pH consistent with experimentally obtained results. J Mol Evol 78:171–187
Kitadai N (2015) Energetics of amino acid synthesis in alkaline hydrothermal environments. Orig Life Evol Biosph. doi:10.1007/s11084-015-9428-3
Lilley TH (1985) Physical properties of amino acid solutions. In: Chemistry and biochemistry of the amino acids. Chapman and Hall, London, pp 591–624
Paukov IE, Kovalevskaya YA, Boldyreva EV, Drebushchak VA (2009) Heat capacity of β-alanine at temperatures of 6 and 300 K. J Therm Anal Calorim 98:873–876
Paukov IE, Kovalevskaya YA, Boldyreva EV (2013) Low-temperature heat capacity and thermodynamic parameters of γ-aminobutyric acid. J Therm Anal Calorim 111:2059–2062
Plyasunov AV, Shock EL (2001) Correlation strategy for determining the parameters of the revised Helgeson–Kirkham–Flowers model for aqueous nonelectrolytes. Geochim Cosmochim Acta 65:3879–3900
Prasad KP, Ahluwalia JC (1976) Heat-capacity changes and partial molal heat capacities of several amino acids in water. J Solut Chem 5:491–507
Price JL, Sorenson EC, Merkley ED, McRae BR, Woolley EM (2003) Thermodynamics of proton dissociation from aqueous L-valine and L-2-amino-n-butanoic acid: apparent molar volumes and apparent molar heat capacities of the protonated cationic, neutral zwitterionic, and deprotonated anionic species at temperatures from 278.15 ≤ T/K ≤ 393.15, at molalities 0.015 ≤ m/mol · kg−1 ≤ 0.67, and pressure p = 0.35 MPa. J Chem Thermodyn 35:1425–1467
Rode BM (1999) Peptides and the origin of life. Peptides 20:773–786
Romero CM, Oviedo CD (2013) Effect of temperature on the solubility of α-amino acids and α, ω-amino acids in water. J Solut Chem 42:1355–1362
Sakata K, Kitadai N, Yokoyama T (2010) Effects of pH and temperature on dimerization rate of glycine: evaluation of favorable environmental conditions for chemical evolution of life. Geochim Cosmochim Acta 74:6841–6851
Schulte MD, Shock EL, Wood RH (2001) The temperature dependence of the standard-state thermodynamic properties of aqueous nonelectrolytes. Geochim Cosmochim Acta 65:3919–3930
Shahidi F (1980) Partial molal volumes of organic compounds in water Part 7.–Sodium and hydrochloride salts of α, ω-aminocarboxylic acids. J Chem Sci Faraday Trans 1 76:101–106
Shahidi F, Farrell PG (1978) Partial molar volumes of organic compounds in water Part 4.–Aminocarboxylic acids. J Chem Sci Faraday Trans 1 74:858–868
Shock EL, Helgeson HC (1990) Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures: standard partial molal properties of organic species. Geochim Cosmochim Acta 54:915–945
Shock EL, Oelkers EH, Johnson JW, Sverjensky DA, Helgeson HC (1992) Calculation of the thermodynamic properties of aqueous species at high pressures and temperatures. J Chem Soc Faraday Trans 88:803–826
Skoulika S, Sabbah R (1983) Thermodynamique de composes azotes. X. Etude thermochimique de quelques acides ω-amines. Thermochim Acta 61:203–214
Smith RM, Martell AE (2004) NIST critically selected stability constants of metal complexes, ver. 8. U. S. Department of Commerce, Technology Administration: U. S. Government Printing Office, Washington, DC
Smith ERB, Smith PK (1942) Thermodynamic properties of solutions of amino acids and related substances VIII. The ionization of glycylglycine, ε-aminocaproic acid, and aspartic acid in aqueous solution from one to fifty degrees. J Biol Chem 146:187–195
Tanger JC, Helgeson HC (1988) Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures: revised equations of state for the standard partial molal properties of ions and electrolytes. Am J Sci 288:19–98
Wang P, Oscarson JL, Gillespie SE, Izatt RM, Cao H (1996) Thermodynamics of protonation of amino acid carboxylate groups from 50 to 125 °C. J Solut Chem 25:243–266
Weber AL, Miller SL (1981) Reasons for the occurrence of the twenty coded protein amino acids. J Mol Evol 17:273–284
Yang XW, Liu JR, Gao SL, Hou YD, Shi QZ (1999) Determination of combustion energies of thirteen amino acids. Thermochim Acta 329:109–115
Zaia DAM, Zaia CTBV, Santana HD (2008) Which amino acids should be used in prebiotic chemistry studies? Orig Life Evol Biosph 38:469–488
Acknowledgments
This manuscript was greatly improved owing to many helpful comments and suggestions from two anonymous reviewers. This research was financially supported by the World Premier International Research Center Initiative (WPI) via my institute (ELSI, Tokyo Tech.).
Author information
Authors and Affiliations
Corresponding author
Appendix 1
Appendix 1
The standard state convention adopted for this study for aqueous species is unit activity of the species in a hypothetical one molal solution referenced to infinite dilution at any temperature or pressure. The conventional standard molal thermodynamic properties of a charged aqueous species are given as the following.
Therein, Ξ and Ξ abs respectively stand for any conventional and absolute standard molal properties of the aqueous species of interest. \( {\Xi}_{H^{+}}^{abs} \) signifies the corresponding absolute standard molal property of the hydrogen ion. In addition, Z represents the charge of the aqueous species of interest.
The standard molal Gibbs energy (∆G o) of aqueous species is expressed as the apparent standard molal Gibbs energy of formation, which is defined as shown below.
Therein, ∆ f G o represents the standard molal Gibbs energy of formation of the species from the elements (O2(g), H2(g), C(graphite), and N2(g)) at the reference temperature (T r = 25 °C) and pressure (P r = 1 bar), and \( {G}_{P,T}^o-{G}_{P_r,{T}_r}^o \) denotes the difference between the standard molal Gibbs energy at temperature (T) and pressure (P) of interest, and that at T r and P r. This term can be evaluated using the following expression.
In that equation, \( {S}_{P_r,{T}_r}^o \) designates the standard molal entropy at T r and P r, \( {C}_{P_r}^o \) represents the standard molal isobaric heat capacity at P r, and V o T denotes the standard molal isothermal volume at the temperature of interest. Consequently, evaluation of the \( {C}_{P_r}^o \) and V o T of aqueous species facilitates calculation of the ∆G o at high temperatures and pressures. This study specifically examines the \( {C}_{P_r}^o \), which allows prediction of the temperature dependence of ∆G o.
Based on the revised HKF equation of state for aqueous species, the isobaric form of the heat capacity is given as (Schulte et al. 2001)
where c 1 , c 2 , and ω represent species-dependent equation-of-state parameters, and where Θ denotes a solvent-dependent parameter equal to 228 K for H2O. In addition, X indicates a Born function defined as shown below.

In that equation, ε stands for the dielectric constant of H2O (Shock et al. 1992).
Temperature dependences of the uncertainties in ∆G o for the non-protein amino acids (Fig. 3) were calculated from the uncertainties in the data and parameters for the respective amino acids presented in Table 2 using the following equation:

where δ denotes uncertainty, and Y represents the Born function defined as  (Shock et al. 1992).
(Shock et al. 1992).
Rights and permissions
About this article
Cite this article
Kitadai, N. Predicting Thermodynamic Behaviors of Non-Protein Amino Acids as a Function of Temperature and pH. Orig Life Evol Biosph 46, 3–18 (2016). https://doi.org/10.1007/s11084-015-9457-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-015-9457-y