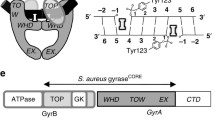
The antibacterial resistance (ABR) is a growing phenomenon and global threat to mankind. To circumvent the ABR, many approaches have been put forth, but none of them meet the pre-requisites associated with the resistance mechanisms. In this review, we focused on the importance of unexploited enzyme, ParE, a topoisomerase responsible for the bacterial survival. The bacterial topoisomerases maintain the topological state of DNA. The gyrases and topoisomerases IV are validated targets for the antibacterial activity. Both these enzymes are structurally similar and possess high degree conservation in the catalytic domain of the N-terminal region, which make them appealing targets for broad spectrum antibacterial activity. Despite being an attractive target for the development of new antibacterials, there are currently no antibiotics targeting gyrases and topoisomerase (topo) IV in the market. Availability of the high-resolution crystal structure data for ParE made it possible to design new classes of antibacterials. Here, we discuss the importance of targeting topo IV enzyme as it is less prone to bacterial resistance which has been disclosed in the literature.








Similar content being viewed by others
References
C. Walsh and G. Wright, ACS Publ., 105 (2), 391 – 394 (2005).
A. Maxwell and D. M. Lawson, Curr. Topics Med. Chem., 3(3), 283 – 303 (2003).
K Drlica and X Zhao, Microbiol. Mol. Biol. Rev., 61(3), 377 – 392 (1997).
P. Heisig, Planta Med., 67(01), 3 – 12 (2001).
H. Peng and K. J. Marians, J. Biol. Chem., 270(42), 25286 – 25290 (1995).
A. B. Khodursky, E. L. Zechiedrich, and N. R. Cozzarelli, Proc. Natl. Acad. Sci. U. S. A., 92, 11801 – 11805 (1995).
M. Takei, H. Fukuda, R. Kishii, et al., Antimicrob. Agents Chemother., 45(12), 3544 – 3547 (2001).
L. Brino, A. Urzhumtsev, M. Mousli, et al., J. Biol. Chem., 275(13), 9468 – 9475 (2000).
L. Brino, C. Bronner, P. Oudet, et al., Biochimie, 81(10), 973 – 980 (1999).
M. Stieger, P. Angehrn, B. Wohlgensinger, et al., Antimicrob. Agents Chemother., 40(4), 1060 – 1062 (1996).
M. Cullen, A. Wyke, R. Kuroda, et al., Antimicrob. Agents Chemother., 33(6), 886 – 894 (1989).
S. Sreedharan, M. Oram, B. Jensen, et al., J. Bacteriol., 172(12), 7260 – 7262 (1990).
R. Hopewell, M. Oram, R. Briesewitz, et al., J. Bacteriol., 172(6), 3481 – 3484 (1990).
R. Changkwanyeun, T. Yamaguchi, S. Kongsoi, et al., Drug Test. Anal., 8(10), 1071 – 1076.
D. C. Stein, R. J. Danaher, and T. M. Cook, Antimicrob. Agents Chemother., 35(4), 622 – 626 (1991).
P. Sparling, F. Sarubbi, and E. Blackman, J. Bacteriol., 124(2), 740 – 749 (1975).
J.-I. Yamagishi, K. Yoshida, M, Yamayoshi, and S. Nakamura, Mol. Gen. Genet., 204, 367 – 373 (1986).
Z. Liao, L. Thibaut, A. Jobson, et al., Mol. Pharmacol., 70(1), 366 – 732 (2006).
M. A. Azam, J. Thathan, and N. S. Tripuraneni, Struct. Chem., 28(4), 1187 – 200 (2017).
F. Sifaoui, V. Lamour, E. Varon, et al., J. Bacteriol., 185(20), 6137 – 6146 (2003).
C. Janoir, V. Zeller, M-D. Kitzis, et al., Antimicrob. Agents Chemother., 40(12), 2760 – 2764 (1996).
A. B. Khodursky and N. R. Cozzarelli, J. Biol. Chem., 273(42), 27668 – 27677 (1998).
S. Bellon, J. D. Parsons, Y. Wei, et al., Antimicrob. Agents Chemother., 48(5), 1856 – 1864 (2004).
Y. Onodera, J. Okuda, M. Tanaka, et al., Antimicrob. Agents Chemother., 46(6), 1800 – 1804 (2002).
P. Angehrn, E. Goetschi, H. Gmuender, et al., J. Med. Chem., 54(7), 2207 – 2224 (2011).
A.-L. Grillot, A. L. Tiran, D. Shannon, et al., J. Med. Chem., 57(21), 8792 – 8816 (2014).
J. T. Starr, R. J. Sciotti, D. L. Hanna, et al., Bioorg. Med. Chem. Lett., 19(18), 5302 – 5306 (2009).
PS Charifson, A-L Grillot, TH Grossman, et al., J. Med. Chem., 51(17), 5243 – 5263 (2008).
G. S. Basarab, J. I. Manchester, S. Bist, et al., J. Med. Chem., 56(21), 8712 – 8735 (2013).
S. P. East, C. B. White, O. Barker, et al., Bioorg. Med. Chem. Lett., 19(3), 894 – 899 (2009).
L. C. Axford, P. K. Agarwal, K. H. Anderson, et al., Bioorg. Med. Chem. Lett., 23(24), 6598 – 6603 (2013).
J. T. Palmer, L. C. Axford, S. Barker, et al., Bioorg. Med. Chem. Lett., 24(17), 4215 – 4222 (2014).
X. Huang, J. Guo, Q. Liu, et al., Med. Chem. Commun., 9(10), 1619 – 1629 (2018).
B. A. Sherer, K. Hull, O. Green, et al., Bioorg. Med. Chem. Lett., 21(24), 7416 – 7420 (2011).
A. Kumar, I. A. Khan, S. Koul, et al., J. Antimicrob. Chemother., 61(6), 1270 – 1276 (2008).
I. A. Yule, L. G. Czaplewski, S. Pommier, et al., Eur. J. Med. Chem., 86, 31 – 38 (2014).
Y. Li, Y. L. Wong, M. Y. Lee, et al., Biomol. NMR Assign., 10(1), 135 – 138 (2016).
Tricyclic Gyrase Inhibitors, US Patent 9732083, August 15 (2017).
Acknowledgements
The authors much acknowledge the All India Council of Technical Education, National Doctoral Fellowship (NDF) Grant-56149 for providing the funding support.
Conflict of Interest
The authors declare that they have no conflict of interest in this study. The authors alone are responsible for the content and writing of the paper.
Ethical Statement
The present study involved neither animals nor human volunteers. Thus, no ethical approval was required.
Author information
Authors and Affiliations
Contributions
Vidyasrilekha Yele was the author and synthesized the literature, involved in drafting the paper; Dr. Afzal Azam Md provided conceptual inputs and critical revision of the manuscript. All authors read and approved the final document.
Corresponding author
Rights and permissions
About this article
Cite this article
Yele, V., Md, A.A. Exploitation of Pare Topoisomerase IV as Drug Target for the Treatment of Multidrug-Resistant Bacteria: A Review. Pharm Chem J 54, 462–468 (2020). https://doi.org/10.1007/s11094-020-02223-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-020-02223-w