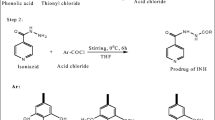
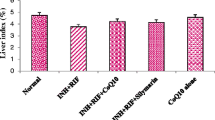
The long-course treatment of tuberculosis with isoniazid (INH) leads to hazardous side effects on liver and poor patient compliance. To overcome these toxic effects caused by INH, a unique hepatoprotective co-drug platform was developed by tethering INH with ursodeoxycholic acid (UDCA) – an antioxidant bile acid for possible synergistic outcome. INH and UDCA were linked into co-drug (UI ) through a bioreversible amide linkage by EDCI coupling. UI resisted hydrolysis in acidic (pH 1.2) and basic (pH 7.4) buffers and stomach homogenate of rat, whereas exhibited significant hydrolysis (83.38%) in intestinal homogenate over a period of 6 h. The effect of UI in attenuating oxidative stress and reinstating the normal physiology of liver was striking as it restored the levels of glutathione peroxidase and superoxide dismutase enzymes to normal. Result obtained for antimycobacterial activity assessment clearly demonstrated that UI was as potent as INH in lowering the mycobacterial load in mice. The outcomes of this exploration confirm that the proposed prodrug can add safety as well as efficiency to future medical procedures of tuberculosis management.






Similar content being viewed by others
References
WHO: Global Tuberculosis Report, World Health Organization Report (2018).
L. A. Kayukova and K.D. Praliev, Pharm. Chem. J., 34, 11 – 18 (2000). https://doi.org/10.1007/BF02524551
I. G. Metushi, P. Cai, X. Zhu, et al., Clin. Pharmacol. Ther., 89, 911 (2011). https://doi.org/10.1038/clpt.2010.355
L. Scharer and J.P. Smith, Ann. Intern. Med., 71, 1113 (1969). https://doi.org/10.7326/0003-4819-71-6-1113.
M. R. Adhvaryu, N. Reddy and B. C. Vakharia, World. J. Gastroenterol., 14, 4753 (2008). https://doi.org/10.3748/wjg.14.4753
N. V. Bhilare, S. S. Dhaneshwar and K. R. Mahadik, Drug. Deliv. Transl. Res., 8, 770 (2018). https://doi.org/10.1007/s13346-018-0500-1
C. Singh, L. Jodave, T. D. Bhatt, et al., Toxicol Rep., 1, 885(2014). https://doi.org/10.1016/j.toxrep.2014.10.001
Z. R. Wu, D. J. Zhi, L. F. Zheng, et al., Med. Chem. Res., 24, 161(2015). https://doi.org/10.1007/s00044-015-1406-9.
N. V. Bhilare, S. S. Dhaneshwar and K. R. Mahadik, World J. Hepatol., 10, 496 (2018). https://doi.org/10.4254/wjh.v10.i7.496
M. Hearn, M. Cynamon, M. Chen, et al., Eur. J. Med. Chem. 2009, 44, 4169. https://doi.org/10.1016/j.ejmech.2009.05.009
K. Horvati, G. Mezo, N. Szabo, et al., J. Pept. Sci., 15, 385 (2009). https://doi.org/10.1002/psc.1129.
R. Cassano, S. Trombino, T. Ferrarelli, et al., Pharm. Pharmacol., 64, 712 (2012). https://doi.org/10.1111/j.2042-7158.2012.01461.x
P. Dandawate, K. Vemuri, K. Venkateswara Swamy, et al., Bioorg. Med. Chem. Lett., 24, 5070 (2014). https://doi.org/10.1016/j.bmcl.2014.09.032
D. Kakkar, A. K. Tiwari, K. Chuttani, et al., Ther. Deliv., 2, 205 (2011). https://doi.org/10.4155/tde.10.97
A. Kumar, G. Patel and S. K. Menon, Chem. Biol. Drug. Des., 73, 553 (2009). https://doi.org/10.1111/j.1747-0285.2009.00804.x
D. Shingnapurkar, P. Dandawate, C. E. Anson, et al., Bioorg. Med. Chem. Lett., 22, 3172 (2012). https://doi.org/10.1016/j.bmcl.2012.03.047
H. Mitsuyoshi, T. Nakashima, Y. Sumida, et al., Biochem. Biophys. Res. Commun., 263, 537 (1999). https://doi.org/10.1006/bbrc.1999.1403
G. A. El-Sherbiny, A. Taye, and I. T. Abdel-Raheem. Ann. Hepatol., 8, 134 (2009). https://doi.org/10.1016/S1665-2681(19)31792-2.
G. Serviddio, J. Pereda, F. V. Pallardo, et al., Hepatology., 39, 711 (2004). https://doi.org/10.1002/hep.20101
X. Chen, J. Xu, C. Zhang, et al., Eur. J. Pharmacol., 659, 53 (2011). https://doi.org/10.1016/j.ejphar.2011.03.007
M. S. Moron, J. W. Depierre and B. Mannervik, Biochim. Biophys. Acta., 582, 67 (1979). https://doi.org/10.1016/0304-4165(79)90289-7
S. Marklund and G. Marklund, Eur. J. Biochem., 47, 469 (1974). https://doi.org/10.1111/j.1432-1033.1974.tb03714.x
S. K. Chanchal, U. B. Mahajan, S. Siddharth, et al., Sci. Rep., 6, 30007 (2016). https://doi.org/10.1038/srep30007
H. Ohkawa, N. Ohishi, and K. Yagi. Anal. Biochem., 95, 351 (1979). https://doi.org/10.1016/0003-2697(79)90738-3
D. R. Sullivan, Z. Kruijswijk, C. E. West, et al., Clin. Chem., 31, 1227 (1985). https://doi.org/10.1093/clinchem/31.7.1227
S. Reitman and S. Frankel, Am. J. Clin. Pathol., 28, 56 (1957). https://doi.org/10.1093/ajcp/28.1.56
Y. Cheng, G. C. Moraski, J. Cramer, et al., PLoS. One., 9, e874 (2014). https://doi.org/10.1371/journal.pone.0087483
I. M. Rosenthal, M. Zhang, K. N. Williams, et al., PLoS. Med., 4, e344 (2007). https://doi.org/10.1371/journal.pmed.0040344
J. Blum and I. Fridovich, Arch. Biochem. Biophys., 240, 500 (1985). https://doi.org/10.1016/0003-9861(85)90056-6
T. A. Fedotchevaa, N. L. Shimanovsky, A. G. Kruglov, et al., Membr. Cell Biol, 6, 92–99 (2012). https://doi.org/10.1134/S1990747811060043
C. Sodhi, S. Rana, S. Mehta, et al., Drug. Chem. Toxicol., 20, 255 (1997). https://doi.org/10.3109/01480549709003881
S. A. Tasduq, K. Peerzada, S. Koul, et al., Hepatol Res., 31, 132 (2005). https://doi.org/10.1016/j.hepres.2005.01.005
B. Bais and P. Saiju, Asian. Pac. J. Trop. Biomed., 4, S633 (2014). https://doi.org/10.12980/APJTB.4.2014APJTB-2014-0236
B. Saxena and S. Sharma, Toxicol. Int., 22, 152 (2015). https://doi.org/10.22506/ti/2015/v22/i3/137623
Z. Tian, H. Liu, X. Su, et al., Exp. Ther. Med., 4, 255 (2012). https://doi.org/10.3892/etm.2012.575
Y. Dong, J. Huang, X. Lin, et al., J. Ethnopharmacol., 27, 201 (2014). https://doi.org/10.1016/j.jep.2014.01.001
R. Pal, S. V. Rana, K. Vaiphei, and K. Singh, Clin. Chim. Acta., 389, 55 (2008). https://doi.org/10.1016/j.cca.2007.11.028
S. Santhosh, T. K. Sini, R. Anandan, and P. T. Mathew, Toxicology, 219, 53 (2006). https://doi.org/10.1016/j.tox.2005.11.001
S. Evan Prince, L. B. Udhaya, P. S. Sunitha and G. Arumugam, Pharmacology, 98, 29 – 34 (2016). https://doi.org/10.1159/000444856
A. Viswanatha Swamy, R. Kulakarni, A. H. M. Thippeswamy, et al.. Indian J. Pharmacol., 42, 397 (2010). https://doi.org/10.4103/0253-7613.71920
V. R. Tandon, V. Khajuria, B. Kapoor, et al., Fitoterapia., 79, 533 (2008). https://doi.org/10.1016/j.fitote.2008.05.005
V. Vijaya Padma, R. Suja and C. S. Shyamala Devi. Fitoterapia., 6, 520 (1998).
X. He, Y. Song, L. Wang, and J. Xu, Mol. Med. Rep., 21, 463 (2020).
S. S. Darvin, S. Esakkimuthu, E. Toppo, et al., Environ. Toxicol. Pharmacol., 61, 87 (2018). https://doi.org/10.1016/j.etap.2018.05.006
E. P. Sabina, S. J. Peter, and A. Geetha, J. Histotechnol., 42, 128 (2019). https://doi.org/10.1080/01478885.2019.1638535
N. V. Bhilare, S. S. Dhaneshwar, A. J. Sinha, et al., Curr. Drug. Deliv., 13, 611(2016). https://doi.org/10.2174/1567201812666150904144607
N. V. Bhilare and S. S. Dhaneshwar, Lett. Drug. Des. Discov., 14, 209 (2017). https://doi.org/10.2174/1570180813666160804152912
L. L. Nwidu and Y. I. Oboma, J. Integr. Med., 17, 46 (2019). https://doi.org/10.1016/j.joim.2018.11.008
T. Y. Shih, T. H. Young, H. S. Lee, et al., AAPS J., 15, 753(2013). https://doi.org/10.1208/s12248-013-9490-6
Y. Ergul, T. Erkan, H. Uzun, et al., Pediatr Int.; 52, 69 (2010). https://doi.org/10.1111/j.1442-200X.2009.02891.x
R. Mohi-Ud-Din, R. Hassan Mir, G. Sawhney, et al., Curr. Drug. Metab., 20, 867 (2019). https://doi.org/10.2174/1389200220666191105121653
Acknowledgments
The authors would like to thankfully acknowledge Lupin Research Park, Lupin Ltd., Aurangabad, India, for providing the gift sample of isoniazid.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhilare, N.V., Dhaneshwar, S.S., Mahadik, K.R. et al. Hepatoprotective Bile Acid Co-Drug of Isoniazid: Synthesis, Kinetics and Investigation of Antimycobacterial Potential. Pharm Chem J 54, 678–688 (2020). https://doi.org/10.1007/s11094-020-02256-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-020-02256-1