Abstract
Purpose
The purpose of this study was to investigate the contribution of drug transporters in acquired imatinib-resistance. Specifically, we focused on the efflux transporters, P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP), and an influx transporter, organic cation transporter 1 (OCT1).
Materials and methods
We established imatinib-resistant K562 cells (K562/IM). Real-time PCR or Western blot analyses were performed to examine the mRNA or protein levels. Alamar blue method was used in the cytotoxicity assay. The transport activities and intracellular imatinib levels were measured by flow cytometry and HPLC, respectively.
Results
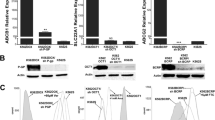
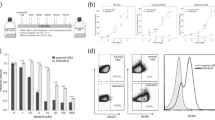
K562/IM displayed a 47-fold increase in resistance to imatinib over the parent K562 cells. Both P-gp and BCRP were overexpressed in K562/IM relative to K562. Furthermore, the intracellular imatinib level was markedly reduced in K562/IM. Interestingly, cyclosporin A, a P-gp inhibitor, but not fumitremorgin C, a BCRP inhibitor, restored both imatinib-sensitivity and the intracellular imatinib level. By contrast, no significant difference in the expression and function of OCT1 was observed between K562/IM and K562.
Conclusions
P-gp, rather than BCRP or OCT1, is partially responsible for the development of imatinib-resistance due to constitutive and functional overexpression, leading to reduced intracellular accumulation of imatinib in K562/IM.



Similar content being viewed by others
Abbreviations
- BCRP:
-
breast cancer resistance protein
- IM:
-
imatinib
- OCT1:
-
organic cation transporter 1
- P-gp:
-
P-glycoprotein
References
M. Kalidas, H. Kantarjian, and M. Talpaz. Chronic myelogenous leukemia. JAMA 286:895–898 (2001).
R. Capdeville, E. Buchdunger, J. Zimmermann, and A. Matter. Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nat. Rev. Drug Discov. 1:493–502 (2002).
B. J. Druker, C. L. Sawyers, H. Kantarjian, D. J. Resta, S. F. Reese, J. M. Ford, R. Capdeville, and M. Talpaz. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N. Engl. J. Med. 344:1038–1042 (2001).
C. L. Sawyers, A. Hochhaus, E. Feldman, J. M. Goldman, C. B. Miller, O. G. Ottmann, C. A. Schiffer, M. Talpaz, F. Guilhot, M. W. N. Deininger, T. Fischer, S. G. O’Brien, R. M. Stone, C. B. Gambacorti-Passerini, N. H. Russell, J. J. Reiffers, T. C. Shea, B. Chapuis, S. Coutre, S. Tura, E. Morra, R. A. Larson, A. Saven, C. Peschel, A. Gratwohl, F. Mandelli, M. Ben-Am, I. Gathmann, R. Capdeville, R. L. Paquette, and B. J. Druker. Imatinib induces hematologic and cytogenetic responses in patients with chronic myelogenous leukemia in myeloid blast crisis: results of a phase II study. Blood 99:3530–3539 (2002).
O. G. Ottmann, B. J. Druker, C. L. Sawyers, J. M. Goldman, J. Reiffers, R. T. Silver, S. Tura, T. Fischer, M. W. Deininger, C. A. Schiffer, M. Baccarani, A. Gratwohl, A. Hochhaus, D. Hoelzer, S. Fernandes-Reese, I. Gathmann, R. Capdeville, and S. G. O’Brien. A phase 2 study of imatinib in patients with relapsed or refractory Philadelphia chromosome-positive acute lymphoid leukemias. Blood 100:1965–1971 (2002).
M. E. Gorre, M. Mohammed, K. Ellwood, N. Hsu, R. Paquette, P. N. Rao, and C. L. Sawyers. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science 293:876–880 (2001).
M. E. O’Dwyer, M. J. Mauro, G. Kurilik, M. Mori, S. Balleisen, S. Olson, E. Magenis, R. Capdeville, and B. J. Druker. The impact of clonal evolution on response to imatinib mesylate (STI571) in accelerated phase CML. Blood 100:1628–1633 (2002).
J. E. Cortes, M. Talpaz, F. Giles, S. O’Brien, M. B. Rios, J. Shan, G. Garcia-Manero, S. Faderl, D. A. Thomas, W. Wierda, A. Ferrajoli, S. Jeha, and H. M. Kantarjian. Prognostic significance of cytogenetic clonal evolution in patients with chronic myelogenous leukemia on imatinib mesylate therapy. Blood 101:3794–3800 (2003).
S. Branford, Z. Rudzki, S. Walsh, A. Grigg, C. Arthur, K. Taylor, R. Herrmann, K. P. Lynch, and T. P. Hughes. High frequency of point mutations clustered within the adenosine triphosphate-binding region of BCR/ABL in patients with chronic myeloid leukemia or Ph-positive acute lymphoblastic leukemia who develop imatinib (STI571) resistance. Blood 99:3472–3475 (2002).
C. Roche-Lestienne, V. Soenen-Cornu, N. Grardel-Duflos, J.-L. Lai, N. Philippe, T. Facon, P. Fenaux, and C. Preudhomme. Several types of mutations of the Abl gene can be found in chronic myeloid leukemia patients resistant to STI571, and they can pre-exist to the onset of treatment. Blood 100:1014–1018 (2002).
N. C. Wolff, D. R. Veach, W. P. Tong, W. G. Bornmann, B. Clarkson, and R. L. Ilaria, Jr. PD166326, a novel tyrosine kinase inhibitor, has greater antileukemic activity than imatinib mesylate in a murine model of chronic myeloid leukemia. Blood 105:3995–4003 (2005).
H. Kantarjian, F. Giles, L. Wunderle, K. Bhalla, S. O’Brien, B. Wassmann, C. Tanaka, P. Manley, P. Rae, W. Mietlowski, K. Bochinski, A. Hochhaus, J. D. Griffin, D. Hoelzer, M. Albitar, M. Dugan, J. Cortes, L. Alland, and O. G. Ottmann. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N. Engl. J. Med. 354:2542–2551 (2006).
M. Talpaz, N. P. Shah, H. Kantarjian, N. Donato, J. Nicoll, R. Paquette, J. Cortes, S. O’Brien, C. Nicaise, E. Bleickardt, M. A. Blackwood-Chirchir, V. Iyer, T.-T. Chen, F. Huang, A. P. Decillis, and C. L. Sawyers. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N. Engl. J. Med. 354:2531–2541 (2006).
F. X. Mahon, M. W. Deininger, B. Schultheis, J. Chabrol, J. Reiffers, J. M. Goldman, and J. V. Melo. Selection and characterization of BCR-ABL positive cell lines with differential sensitivity to the tyrosine kinase inhibitor STI571: diverse mechanisms of resistance. Blood 96:1070–1079 (2000).
N. J. Donato, J. Y. Wu, J. Stapley, G. Gallick, H. Lin, R. Arlinghaus, and M. Talpaz. BCR-ABL independence and LYN kinase overexpression in chronic myelogenous leukemia cells selected for resistance to STI571. Blood 101:690–698 (2003).
Y. Dai, M. Rahmani, S. J. Corey, P. Dent, and S. Grant. A Bcr/Abl-independent, Lyn-dependent form of imatinib mesylate (STI-571) resistance is associated with altered expression of Bcl-2. J. Biol. Chem. 279:34227–34239 (2004).
F.-X. Mahon, F. Belloc, V. Lagarde, C. Chollet, F. Moreau-Gaudry, J. Reiffers, J. M. Goldman, and J. V. Melo. MDR1 gene overexpression confers resistance to imatinib mesylate in leukemia cell line models. Blood 101:2368–2373 (2003).
A. Hamada, H. Miyano, H. Watanabe, and H. Saito. Interaction of imatinib mesilate with human P-glycoprotein. J. Pharmacol. Exp. Ther. 307:824–828 (2003).
N. Widmer, S. Colombo, T. Buclin, and L. A. Decosterd. Functional consequence of MDR1 expression on imatinib intracellular concentrations. Blood 102:1142
T. Illmer, M. Schaich, U. Platzbecker, J. Freiberg-Richter, U. Oelschlagel, M. von Bonin, S. Pursche, T. Bergemann, G. Ehninger, and E. Schleyer. P-glycoprotein-mediated drug efflux is a resistance mechanism of chronic myelogenous leukemia cells to treatment with imatinib mesylate. Leukemia 18:401–408 (2004).
A. Radujkovic, M. Schad, J. Topaly, M. R. Veldwijk, S. Laufs, B. S. Schultheis, A. Jauch, J. V. Melo, S. Fruehauf, and W. J. Zeller. Synergistic activity of imatinib and 17-AAG in imatinib-resistant CML cells overexpressing BCR-ABL—inhibition of P-glycoprotein function by 17-AAG. Leukemia 19:1198–1206 (2005).
M. Mukai, X.-F. Che, T. Furukawa, T. Sumizawa, S. Aoki, X.-Q. Ren, M. Haraguchi, Y. Sugimoto, M. Kobayashi, H. Takamatsu, and S.-i. Akiyama. Reversal of the resistance to STI571 in human chronic myelogenous leukemia K562 cells. Cancer Sci. 94:557–563 (2003).
F. J. Giles, H. Kantarjian, J. Cortes, D. Thomas, M. Talpaz, T. Manshouri, and M. Albitar. Multidrug resistance protein expression in chronic myeloid leukemia: Associations and significance. Cancer 86:805–813 (1999).
H. Burger, H. van Tol, A. W. Boersma, M. Brok, E. A. Wiemer, G. Stoter, and K. Nooter. Imatinib mesylate (STI571) is a substrate for the breast cancer resistance protein (BCRP)/ABCG2 drug pump. Blood 104:2940–2942 (2004).
P. Breedveld, D. Pluim, G. Cipriani, P. Wielinga, O. van Tellingen, A. H. Schinkel, and J. H. M. Schellens. The Effect of Bcrp1 (Abcg2) on the in vivo pharmacokinetics and brain penetration of imatinib mesylate (Gleevec): implications for the use of breast cancer resistance protein and P-glycoprotein inhibitors to enable the brain penetration of imatinib in patients. Cancer Res. 65:2577–2582 (2005).
P. J. Houghton, G. S. Germain, F. C. Harwood, J. D. Schuetz, C. F. Stewart, E. Buchdunger, and P. Traxler. Imatinib mesylate is a potent inhibitor of the ABCG2 (BCRP) transporter and reverses resistance to topotecan and SN-38 in vitro. Cancer Res. 64:2333–2337 (2004).
N. E. Jordanides, H. G. Jorgensen, T. L. Holyoake, and J. C. Mountford. Functional ABCG2 is overexpressed on primary CML CD34+ cells and is inhibited by imatinib mesylate. Blood 108:1370–1373 (2006).
J. Thomas, L. Wang, R. E. Clark, and M. Pirmohamed. Active transport of imatinib into and out of cells: implications for drug resistance. Blood 104:3739–3745 (2004).
L. C. Crossman, B. J. Druker, M. W. N. Deininger, M. Pirmohamed, L. Wang, and R. E. Clark. hOCT 1 and resistance to imatinib. Blood 106:1133–1134 (2005).
D. L. White, V. A. Saunders, P. Dang, J. Engler, A. C. Zannettino, A. C. Cambareri, S. R. Quinn, P. W. Manley, and T. P. Hughes. OCT-1-mediated influx is a key determinant of the intracellular uptake of imatinib but not nilotinib (AMN107): reduced OCT-1 activity is the cause of low in vitro sensitivity to imatinib. Blood 108:697–704 (2006).
H. M. Kantarjian, M. Talpaz, S. O’Brien, F. Giles, G. Garcia-Manero, S. Faderl, D. Thomas, J. Shan, M. B. Rios, and J. Cortes. Dose escalation of imatinib mesylate can overcome resistance to standard-dose therapy in patients with chronic myelogenous leukemia. Blood 101:473–475 (2003).
J. O’Brien, I. Wilson, T. Orton, and F. Pognan. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 267:5421–5426 (2000).
G. W. Dewald, W. A. Wyatt, A. L. Juneau, R. O. Carlson, A. R. Zinsmeister, S. M. Jalal, J. L. Spurbeck, and R. T. Silver. Highly sensitive fluorescence in situ hybridization method to detect double BCR/ABL fusion and monitor response to therapy in chronic myeloid leukemia. Blood 91:3357–3365 (1998).
R. W. Robey, K. Steadman, O. Polgar, K. Morisaki, M. Blayney, P. Mistry, and S. E. Bates. Pheophorbide a is a specific probe for ABCG2 function and inhibition. Cancer Res. 64:1242–1246 (2004).
C. Özvegy-Laczka, T. Hegedüs, G. Varady, O. Ujhelly, J. D. Schuetz, A. Varadi, G. Keri, L. Örfi, K. Nemet, and B. Sarkadi. High-affinity interaction of tyrosine kinase inhibitors with the ABCG2 multidrug transporter. Mol. Pharmacol. 65:1485–1495 (2004).
H. Burger, H. van Tol, M. Brok, E. A. Wiemer, E. A. de Bruijn, G. Guetens, G. de Boeck, A. Sparreboom, J. Verweij, and K. Nooter. Chronic imatinib mesylate exposure leads to reduced intracellular drug accumulation by induction of the ABCG2 (BCRP) and ABCB1 (MDR1) drug transport pumps. Cancer Biol. Ther. 4:747–752 (2005).
T. Nakanishi, K. Shiozawa, B. A. Hassel, and D. D. Ross. Complex interaction of BCRP/ABCG2 and imatinib in BCR-ABL-expressing cells: BCRP-mediated resistance to imatinib is attenuated by imatinib-induced reduction of BCRP expression. Blood 108:678–684 (2006).
E. R. Gardner, H. Burger, R. H. van Schaik, A. T. van Oosterom, E. A. de Bruijn, G. Guetens, H. Prenen, F. A. de Jong, S. D. Baker, S. E. Bates, W. D. Figg, J. Verweij, A. Sparreboom, and K. Nooter. Association of enzyme and transporter genotypes with the pharmacokinetics of imatinib. Clin. Pharmacol. Ther. 80:192–201 (2006).
M. Warmuth, N. Simon, O. Mitina, R. Mathes, D. Fabbro, P. W. Manley, E. Buchdunger, K. Forster, I. Moarefi, and M. Hallek. Dual-specific Src and Abl kinase inhibitors, PP1 and CGP76030, inhibit growth and survival of cells expressing imatinib mesylate-resistant Bcr-Abl kinases. Blood 101:664–672 (2003).
Acknowledgments
We thank Dr. Robert W. Robey and Dr. Susan E. Bate at National Cancer Institute (Betheda, MD) for providing FTC and PhA. We also acknowledge Novartis Pharma AG (Basel, Switzerland) for kindly providing imatinib and cyclosporin A.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hirayama, C., Watanabe, H., Nakashima, R. et al. Constitutive Overexpression of P-glycoprotein, Rather than Breast Cancer Resistance Protein or Organic Cation Transporter 1, Contributes to Acquisition of Imatinib-Resistance in K562 Cells. Pharm Res 25, 827–835 (2008). https://doi.org/10.1007/s11095-007-9376-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9376-3