Abstract
Background
Polysaccharides constituting about 10% by weight of ginseng root are known to stimulate the immune system but have recently been shown to also suppress induced proinflammatory responses. Our study aims to determine whether American ginseng root polysaccharides (AGRPS) stimulates basal innate immune function and at the same time can suppress response to lipopolysaccharide (LPS) induced proinflammatory response. An in vitro mechanistic study was used to identify the bioactive fraction(s) responsible for AGRPS immunomodulatory effects.
Methods
The ex vivo and in vivo immunomodulatory effects after oral administration of AGRPS extract was studied in adult rats by measuring cultured alveolar macrophage production of NO and changes of plasma cytokine level, modification of LPS proinflammatory immune response by AGRPS extract was also examined. To identify the bioactive fraction(s) responsible for AGRPS extract immunomodulatory effects, the immunobioactivities of the extract fractions (isolated by ion exchange and size exclusion chromatography) was investigated in an in vitro mechanistic study.
Results
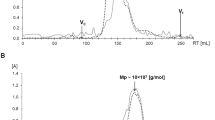
Culture of alveolar macrophages obtained from AGRPS extract treated rats resulted in an increase of ex vivo production of NO and also reduced alveolar macrophage responsiveness to ex vivo LPS challenge. Oral treatment with AGRPS extract elevated plasma TNF-α concentration in vivo. This treatment also suppressed LPS induced elevation of plasma TNF-α in vivo. AGRPS extract immunostimulatory and immunouppressive effects were mediated primarily by acid PS and its species with molecular weights ≥100 kDa and 50–100 kDa.
Conclusion
: AGRPS extract exerted immunostimulation and suppressed LPS immune response under basal and LPS induced proinflammatory conditions respectively.















Similar content being viewed by others
References
Wood PJ. The immune system: recognition of infectious agents. Anaesthesia and Intensive Care Medicine. 2006;7:179–80.
Chung HS, Kang M, Cho C, Park S, Kim H, Yoon YS, et al. Inhibition of lipopolysaccharide and interferon-gamma-induced expression of inducible nitric oxide synthase and tumor necrosis factor-alpha by Lithospermi radix in mouse peritoneal macrophages. Journal of Ethnopharmacology. 2005;102:412–7.
Mee-Young L, Bo-Young Park A, Ok-Kyoung Kwon A, Ji-Eun Y, Sei-Ryang Oh A, Hui-Seong K, et al. Anti-inflammatory activity of (−)-aptosimon isolated from Daphne genkwa in RAW264.7. International Immunopharmacology. 2009;9:878–85.
Schletter J, Heine H, Ulmer AJ, Rietschel ET. Molecular mechanisms of endotoxin activity. Archives of Microbiology. 1995;164:383–9.
Nakamura A, Mori Y, Hagiwara K, Suzuki T, Sakakibara T, Kikuchi T, et al. Increased susceptibility to LPS-induced endotoxin shock in secretory leukoprotease inhibitor (SLPI)-deficient mice. Journal of Experimental Medicine. 2003;3:669–74.
Sireci G, La Manna MP, Di Liberto D, Lo Dico M, Taniguchi M, Dieli F, et al. Prophylaxis of lipopolysaccharide-induced shock by alpha-galactosylceramide. Journal of Leukocyte Biology. 2008;84:550–60.
Szabo C. Alterations in nitric oxide production in various forms of circulatory shock. New Horizons. 1995;3:2–32.
Beumer C, Wulferink M, Raaben W, Fiechter D, Brands R, Seinen W. Calf Intestinal alkaline phosphatase, a novel therapeutic drug for lipopolysaccharide (LPS)- mediated diseases, attenuates LPS toxicity in mice and piglets. The Journal of Pharmacology and Experimetal Therapeutics. 2003;307:737–44.
Danner RL, Joiner KA, Rubin M, Patterson WH, Johnson N, Ayers KM, et al. Purification, toxicity, and antiendotoxin activity of polymyxin B nonapeptide. Antimicrobial Agents and Chemotherapy. 1989;33:1428–34.
Schepetkin IA, Quinn MT. Botanical polysaccharides: macrophage immunomodulation and therapeutic potential. International Immunopharmacology. 2006;6:317–33.
Tzianabos AO. Polysaccharide immunomodulators as therapeutic agents: structural aspects and biologic function. Clinical Microbiology Reviews. 2000;13:523–33.
Shao D, Dunlop WD, Lui MK, Bernards MA. Immunostimulatory and anti- inflammatory polysaccharides from tripterygium wilfordii: comparison with organic extracts. Pharmaceutical Biology. 2008;46:8–15.
Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochemical Pharmacology. 1999;58:1685–93.
Angelova N, Kong HW, van der Heijden R, Yang SY, Choi YH, Kim HK, et al. Recent methodology in the phytochemical analysis of ginseng. Phytochemical Analysis. 2008;19:2–16.
Lim TS, Na K, Choi EM, Chung JY, Hwang JK. Immunomodulating activities of polysaccharides isolated from Panax ginseng. Journal of Medicinal Food. 2004;7:1–6.
Kim YS, Kang SK, Kim S. Study on antitumor and immunomodulating activities of polysaccharide fractions from Panax ginseng: comparison of effects of neutral and acidic polysaccharide fraction. Archives of Pharmacal Research. 1990;13:330–7.
Luo D, Fang B. Structural identification of ginseng polysaccharides and testing of their antioxidant activities. Carbohydrate Polymers. 2008;72:376–81.
Wang J, Flaisher-Grinberg S, Li S, Liu H, Sun L, Zhou Y, et al. Antidepressant- like effects of the active acidic polysaccharide portion of ginseng in mice. Journal of Ethnopharmacology. 2010;132:65–9.
Xie JT, Wu JA, Mehendale S, Aung HH, Yuan CS. Anti-hyperglycemic effect of the polysaccharides fraction from American ginseng berry extract in ob/ob mice. Phytomedicine. 2004;11:182–7.
Srivastava R, Kulshreshtha D. Bioactive polysaccharides from plants. Phytochemistry. 1989;28:2877–83.
Biondo PD, Goruk S, Ruth MR, O’Connell E, Field CJ. Effect of CVT-E002™ (COLD-FX®) versus a ginsenoside extract on systemic and gut-associated immune function. International Immunopharmacology. 2008;8:1134–42.
Wang M, Guilbert LJ, Li J, Wu Y, Pang P, Basu TK, et al. A proprietary extract from North American ginseng (Panax quinquefolium) enhances IL-2 and IFN-gamma productions in murine spleen cells induced by Con-A. International Immunopharmacology. 2004;4:311–5.
Lui EMK, Azike CG, Guerrero-Analco JA, Kalda SJ, Romeh AA, Pei H, et al. Bioactive polysaccharides of American Ginseng Panax quinquefolius L. in Modulation of Immune Function: phytochemical and pharmacological characterization. the complex world of polysaccharides. Intech publications. Chapter. 2012;19:513–34.
Zhang X, Li Y, Bi HB, Li XH, Ni WH, Han H, et al. Total fractionation and characterization of the water-soluble polysaccharides isolated from Panax ginseng C. A. Meyer. Carbohydrate Polymers. 2009;77:544–52.
Assinewe VA, Arnason JT, Aubry A, Mullin J, Lemaire I (2002). Extractable Polysaccharides of Panax quinquefolius L. (North American ginseng) root stimulate TNF-α production by alveolar macrophages. Phytomedicine, 9, 398–404.
Sun Y. Structure and biological activities of the polysaccharides from the leaves, roots and fruits of Panax ginseng C.A. meyer: an overview. Carbohydrate Polymers. 2011;85:490–9.
Tomoda M, Hirabayashi K, Shimizu N, Gonda R, O’hara N, Takada K. Characterization of two novel polysaccharides having immunological activities from the root of Panax ginseng. Biological & Pharmaceutical Bulletin. 1993;16:1087–90.
Sonoda Y, Kasaharaa T, Mukaida N, Shimizua N, Tomoda M, Takeda T. Stimulation of interleukin-8 production by acidic polysaccharides from the root of Panax ginseng. Immunopharmacology. 2006;38:287–94.
Wang M, Guilbert LJ, Ling L, Li J, Wu Y, Xu S, et al. Immunomodulating activity of CVT-E002, a proprietary extract from North American ginseng (Panax quinquefolium). Journal of Pharmacy and Pharmacology. 2001;53:1515–23.
Friedl R, Moeslinger T, Kopp B, Spieckermann PG. Stimulation of nitric oxide synthesis by the aqueous extract of Panax ginseng root in RAW 264.7 cells. British Journal of Pharmacology. 2001;134:1663–70.
Choi HS, Kim KH, Sohn E, Park JD, Kim BO, Moon EY, et al. Red ginseng acidic polysaccharide (RGAP) in combination with IFN-gamma results in enhanced macrophage function through activation of the NF-kappaB pathway. Bioscience Biotechnology and Biochemistry. 2008;72:1817–25.
Azike CG, Charpentier PA, Hou J, Pei H, Lui EMK. The yin and yang actions of North American ginseng root in modulating the immune function of macrophages. Chinese Medicine. 2011;6:1–12.
Zhao H, Zhang W, Xiao C, Lu C, Xu S, He X, et al. Effect of ginseng polysaccharide on TNF-α and INF- γ produced by enteric mucosal lymphocytes in collagen Induced arthritic rats. Journal of Medicinal Plants Research. 2011;5:1536–42.
Ahn JY, Choi IS, Shim JY, Yun EK, Yun YS, Jeong G, et al. The Immunomodulator ginsan induces resistance to experimental sepsis by inhibiting Toll-like receptor-mediated inflammatory signals. European Journal of Immunology. 2006;36:37–45.
Ahn JY, Song JY, Yun YS, Jeong G, Choi IS. Protection of Staphylococcus aureus-infected septic mice by suppression of early acute inflammation and enhanced antimicrobial activity by ginsan. FEMS Immunology and Medical Microbiology. 2006;46:187–97.
Yoo DG, Kim MC, Park MK, Park KM, Quan FS, Song JM, et al. Protective effect of ginseng polysaccharides on influenza viral infection. PloS One. 2012;7:e33678.
Tao Y, Zhang L, Cheung PC. Physicochemical properties and antitumor activities of water-soluble native and sulfated hyperbranched mushroom polysaccharides. Carbohydrate Research. 2006;341:2261–9.
Lin Y, Zhang L, Chen L, Jin Y, Zeng F, Jin J, et al. Molecular mass and antitumor activities of sulfated derivatives of alpha-glucan from Poria cocos mycelia. International Journal of Biological Macromolecules. 2004;34:289–94.
Wang Y, Zhang M, Ruan D, Shashkov AS, Kilcoyne M, Savage AV, et al. Chemical components and molecular mass of six polysaccharides isolated from the Sclerotium of Poria cocos. Carbohydrate Research. 2004;339:327–34.
Sevag MG, Lackman DB, Smolens J. The isolation of the components of streptococcal nucleoproteins in serologically active form. Journal of Biological Chemistry. 1938;124:425–36.
Copeland S, Warren HS, Lowry SF, Calvano SE, Remick D. Acute inflammatory response to endotoxin in mice and humans. Clinical and Diagnostic Laboratory Immunology. 2005;12:60–7.
Jiang J, Bahrami S, Leichtfried G, Redl H, Ohlinger W, Schlag G. Kinetics of endotoxin and tumor necrosis factor appearance in portal and systemic circulation after hemorrhagic shock in rats. Annals Surgery. 1995;221:100–6.
Altavilla D, Squadrito F, Serrano M, Campo GM, Squadrito G, Arlotta M, et al. Inhibition of tumour necrosis factor and reversal of endotoxin- induced shock by U-83836E, a ‘second generation’ lazaroid in rats. British Journal of Pharmacology. 1998;124:1293–9.
Fernández-Martínez E, Morales-Ríos MS, Pérez-Alvarez V, Muriel P. Immunomodulatory effects of thalidomide analogs on LPS-induced plasma and hepatic cytokines in the rat. Biochememical Pharmacology. 2004;68:1321–9.
Nezić L, Skrbić R, Dobrić S, Stojiljković MP, Satara SS, Milovanović ZA, et al. Effect of simvastatin on proinflammatory cytokines production during lipopolysaccharide-induced inflammation in rats. General Physiology and Biophysics. 2009;28:119–26.
Song JY, Han SK, Son EH, Pyo SN, Yun YS, Yi SY. Induction of secretory and tumoricidal activities in peritoneal macrophages by ginsan. International Immunopharmacology. 2002;2:857–65.
He X, Niu X, Li J, Xu S, Lu A. Immunomodulatory activities of five clinically used chinese herbal polysaccharides. Journal of Experimental and Integrative Medicine. 2012;2:15–27.
Predy GN, Goel V, Lovlin RE, Basu TK. Immune modulating effects of daily supplementation of COLD-FX (a proprietary extract of North American ginseng) in healthy adults. Journal of Clinical Biochemistry and Nutrition. 2006;39:162–7.
Giebelen A, VanWesterloo DJ, LaRosa GJ, De Vos AF, Van der Poll T. Local stimulation of α7 cholinergic receptors inhibits LPS-induced TNF-α release in the mouse lung. Shock. 2007;28:700–3.
Sugiyama Y. Preventive effect of Hochu-ekki-to on lipopolysaccharide-induced acute lung injury in BALB/c mice. Lung. 2006;184:318–23.
Manjeet KR, Ghosh B. Quercetin inhibits LPS-induced nitric oxide and tumor necrosis factor-alpha production in murine macrophages. International Journal of Immunopharmacology. 1999;21:435–43.
Ni W, Zhang X, Wang B, Chen Y, Han H, Fan Y, et al. Antitumor activities and immunomodulatory effects of ginseng neutral polysaccharides in combination with 5-fluorouracil. Journal of Medicinal Food. 2010;13:270–7.
Lemmon HR, Sham J, Chau LA, Madrenas J. High molecular weight polysaccharides are key immunomodulators in North American ginseng extracts: characterization of the ginseng genetic signature in primary human immune cells. Journal of Ethnopharmacology. 2012;14:1–13.
Ando I, Tsukumo Y, Wakabayashi T, Akashi S, Miyake K, Kataoka T, et al. Safflower polysaccharides activate the transcription factor NF-kappa B via Toll-like receptor 4 and induce cytokine production by macrophages. International Immunopharmacology. 2002;2:1155–62.
Yoon YD, Han SB, Kang JS, Lee CW, Park SK, Lee HS, et al. Toll- like receptor 4-dependent activation of macrophages by polysaccharide isolated from the radix of Platycodon grandiflorum. International Immunopharmacology. 2003;3:1873–82.
Xu Q, Yajima T, Li W, Saito K, Ohshima Y, Yoshikai Y. Levan (beta-2, 6-fructan), a major fraction of fermented soybean mucilage, displays immunostimulating properties via Toll-like receptor 4 signalling: induction of interleukin-12 production and suppression of T- helper type 2 response and immunoglobulin E production. Clinical & Experimental Allergy. 2006;36:94–101.
Zhu Y, Pettolino F, Mau SL, Shen YC, Chen CF, Kuo YC, et al. Immunoactive polysaccharide-rich fractions from Panax notoginseng. Planta Medica. 2006;72:1193–9.
Leon-Ponte M, Kirchhof MG, Sun T, Stephens T, Singh B, Sandhu S, et al. Polycationic lipids inhibit the pro-inflammatory response to LPS. Immunology Letters. 2005;96:73–83.
High KP, Case D, Hurd D, Powell B, Lesser G, Falsey AR, et al. A randomized, controlled trial of Panax quinquefolius extract (CVT-E002) to reduce respiratory infection in patients with chronic lymphocytic leukemia. The Journal of Supportive Oncology. 2012;10:195–201.
Miller SC, Delorme D, Shan JJ. CVT-E002 stimulates the immune system and extends the life span of mice bearing a tumor of viral origin. Journal of the Society for Integrative Oncology. 2009;7:127–36.
Miller SC, Delorme D, Shan JJ. Extract of North American ginseng (Panax quinquefolius), administered to leukemic, juvenile mice extends their life span. Journal of Complementary and Integrative Medicine. 2011;8:1–10.
Miller SC, Ti L, Shan JJ. The sustained influence of short term exposure to a proprietary extract of North American ginseng on the hemopoietic cells of the bone marrow, spleen and blood of adult and juvenile mice. Phytotherapy Research. 2012;26:675–81.
Ebeling C, Wu Y, Skappak C, Gordon JR, Ilarraza R, Adamko DJ. Compound CVT- E002 attenuates allergen-induced airway inflammation and airway hyperresponsiveness, in vivo. Molecular Nutrition & Food Research. 2011;55:1905–8.
Eichacker PQ, Parent C, Kalil A, Esposito C, Cui X, Banks SM, et al. Risk and the efficacy of anti-inflammatory agents: retrospective and confirmatory studies of sepsis. American Journal of Respiratory and Critical Care Medicine. 2002;166:1197–205.
Vincent JL, Sun Q, Dubois MJ. Clinical trials of immunomodulatory therapies in severe sepsis and septic shock. Clinical Infectious Diseases. 2002;34:1084–93.
Opal SM, Gluck T. Endotoxin as a drug target. Critical Care Medicine. 2003;31:57–64.
Azike CG, Charpentier PA, Xu ZW, Romeh AA, Lui EMK (2014). Analysis of Intestinally Absorbed American Ginseng Polysaccharides in Plasma by High Performance Size Exclusion Chromatography. Manuscript in preparation.
Lin X, Wang Z, Sun G, Shen L, Xu D, Feng Y. A sensitive and specific HPGPC- FD method for the study of pharmacokinetics and tissue distribution of Radix Ophiopogonis polysaccharide in rats. Biomedical Chromatography. 2010;24:820–5.
The State Pharmacopoeia Commission of the People’s Republic of China. Pharmacopoeia of the People’s Republic of China (English edition). China: Chemical Industry Press; 2000.
Chan K. Chinese medicinal materials and their interface with Western medical concepts. Journal of Ethnopharmacology. 2005;96:1–18.
Tsao R, Liu Z. Exploration and characterization of bioactive phytochemicals in native Canadian plants for human health. Canadian Journal of Plant Science. 2007;87:1045–53.
Acknowledgments
This research was supported by Ontario Ginseng Research & Innovation Consortium (OGRIC) funded by the Ministry of Research & Innovation, Ontario Research Funded Research Excellence program for the project ‘New Technologies for Ginseng Agriculture and Product Development’(RE02-049 awarded to EMK Lui).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Azike, C.G., Charpentier, P.A. & Lui, E.M. Stimulation and Suppression of Innate Immune Function by American Ginseng Polysaccharides: Biological Relevance and Identification of Bioactives. Pharm Res 32, 876–897 (2015). https://doi.org/10.1007/s11095-014-1503-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1503-3