Abstract
Purpose
To address the problem of precipitation of a poorly soluble drug during dissolution of highly soluble cocrystals by preparing granules intimately mixed with a water-soluble polymer.
Methods
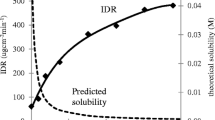
Effectiveness of polymers as precipitation inhibitors during the dissolution of carbamazepine–nicotinamide (CBZ-NCT) cocrystal was assessed based on induction time of crystallization from a supersaturated solution in presence of different polymers at two concentrations. Dissolution was evaluated by both intrinsic dissolution rate (IDR) and USP dissolution method. Powder manufacturability was assessed using a shear cell and compaction simulator to assess flowability and tabletability, respectively.
Results
Hydroxypropyl methylcellulose acetate succinate (HPMCAS) was the most effective polymer against precipitation of CBZ and the IDR of a 1:1 (w/w) CBZ-NCT/HPMCAS mixture was the highest. The final formulation of 1:1 CBZ-NCT/HPMCAS granule exhibited excellent flowability, good tabletability, and significantly improved drug release rate than cocrystal formulations without HPMCAS or the CBZ formulation.
Conclusion
The particle engineering strategy of modifying the diffusion layer on the surface of highly soluble cocrystal with a polymer is effective for inhibiting premature precipitation of CBZ. Assisted with predictive tools for characterizing powder flowability and tabletability, the design of high quality tablet product with improved drug release rate and manufacturability can be achieved in an efficient manner.







Similar content being viewed by others
Abbreviations
- ASD:
-
Amorphous solid dispersion
- CBZ:
-
Carbamazepine
- HPMCAS:
-
Hydroxypropyl methylcellulose acetate succinate
- IDR:
-
Intrinsic dissolution rate
- NCT:
-
Nicotinamide
- PXRD:
-
Powder X-ray diffraction pattern
References
McNamara DP, Childs SL, Giordano J, Iarriccio A, Cassidy J, Shet MS, et al. Use of a glutaric acid cocrystal to improve oral bioavailability of a low solubility API. Pharm Res. 2006;23:1888–97.
Karki S, Friscic T, Fabian L, Laity PR, Day GM, Jones W. Improving mechanical properties of crystalline solids by Cocrystal formation: new compressible forms of paracetamol. Adv Mater. 2009;21:3905–9.
Aakeroy CB, Forbes S, Desper J. Using Cocrystals to systematically modulate aqueous solubility and melting behavior of an anticancer drug. J Am Chem Soc. 2009;131:17048–9.
Sun CC, Hou H. Improving mechanical properties of caffeine and methyl gallate crystals by cocrystallization. Cryst Growth Des. 2008;8:1575–9.
Duggirala NK, Perry ML, Almarsson O, Zaworotko MJ. Pharmaceutical cocrystals: along the path to improved medicines. Chem Commun. 2016;52:640–55.
Sun CC. Cocrystallization for successful drug delivery. Expert Opinion on Drug Delivery. 2013;10:201–13.
Tahara A, Kurosaki E, Yokono M, Yamajuku D, Kihara R, Hayashizaki Y, et al. Antidiabetic effects of SGLT2-selective inhibitor Ipragliflozin in Streptozotocin-nicotinamide-induced mildly diabetic mice. J Pharmacol Sci. 2012;120:36–44.
Thipparaboina R, Kumar D, Chavan RB, Shastri NR. Multidrug co-crystals: towards the development of effective therapeutic hybrids. Drug Discov Today. 2016;21:481–90.
Takata N, Shiraki K, Takano R, Hayashi Y, Terada K. Cocrystal screening of stanolone and mestanolone using slurry crystallization. Cryst Growth Des. 2008;8:3032–7.
Jayasankar A, Reddy LS, Bethune SJ, Rodriguez-Hornedo N. Role of Cocrystal and solution chemistry on the formation and stability of Cocrystals with different stoichiometry. Cryst Growth Des. 2009;9:889–97.
Zhang GGZ, Henry RF, Borchardt TB, Lou X. Efficient co-crystal screening using solution-mediated phase transformation. J Pharm Sci. 2007;96:990–5.
Weyna DR, Shattock T, Vishweshwar P, Zaworotko MJ. Synthesis and structural characterization of Cocrystals and pharmaceutical Cocrystals: Mechanochemistry vs slow evaporation from solution. Cryst Growth Des. 2009;9:1106–23.
Trask AV, van de Streek J, Motherwell WDS, Jones W. Achieving polymorphic and stoichiometric diversity in cocrystal formation: importance of solid-state grinding, powder X-ray structure determination, and seeding. Cryst Growth Des. 2005;5:2233–41.
Yamashita H, Hirakura Y, Yuda M, Teramura T, Terada K. Detection of Cocrystal formation based on binary phase diagrams using thermal analysis. Pharm Res-Dordr. 2013;30:70–80.
Yamashita H, Hirakura Y, Yuda M, Terada K. Coformer screening using thermal analysis based on binary phase diagrams. Pharm Res. 2014;31:1946–57.
Alhalaweh A, Ai HRH, Velaga SP. Effects of polymer and surfactant on the dissolution and transformation profiles of Cocrystals in aqueous media. Cryst Growth Des. 2014;14:643–8.
Childs SL, Kandi P, Lingireddy SR. Formulation of a Danazol Cocrystal with controlled supersaturation plays an essential role in improving bioavailability. Mol Pharm. 2013;10:3112–27.
Qiu S, Li M. Effects of coformers on phase transformation and release profiles of carbamazepine cocrystals in hydroxypropyl methylcellulose based matrix tablets. Int J Pharm. 2015;479:118–28.
Ullah M, Hussain I, Sun CC. The development of carbamazepine-succinic acid cocrystal tablet formulations with improved in vitro and in vivo performance. Drug Dev Ind Pharm. 2016;42:969–76.
Good DJ, Rodriguez-Hornedo N. Solubility advantage of pharmaceutical Cocrystals. Cryst Growth Des. 2009;9:2252–64.
Lipert MP, Rodriguez-Hornedo N. Cocrystal transition points: role of Cocrystal solubility, drug solubility, and solubilizing agents. Mol Pharm. 2015;12:3535–46.
Yamashita H, Sun CC. Harvesting potential dissolution advantages of soluble Cocrystals by depressing precipitation using the common Coformer effect. Cryst Growth Des. 2016;16:6719–21.
Yamashita H, Sun CC. Improving dissolution rate of carbamazepine-Glutaric acid Cocrystal through Solubilization by excess Coformer. Pharm Res-Dordr. 2018;35.
Yamashita H, Sun CC. Self-templating accelerates precipitation of carbamazepine dihydrate during the dissolution of a soluble carbamazepine cocrystal. CrystEngComm. 2017;19:1156–9.
Issa MG, Ferraz HG. Intrinsic dissolution as a tool for evaluating drug solubility in accordance with the biopharmaceutics classification system. Dissolut Technol. 2011;18:6–13.
Hawley M, Morozowich W. Modifying the diffusion layer of soluble salts of poorly soluble basic drugs to improve dissolution performance. Mol Pharm. 2010;7:1441–9.
Sehic S, Betz G, Hadzidedic S, El-Arini SK, Leuenberger H. Investigation of intrinsic dissolution behavior of different carbamazepine samples. Int J Pharm. 2010;386:77–90.
Yu LX, Carlin AS, Amidon GL, Hussain AS. Feasibility studies of utilizing disk intrinsic dissolution rate to classify drugs. Int J Pharm. 2004;270:221–7.
Dokoumetzidis A, Macheras P. A century of dissolution research: from Noyes and Whitney to the biopharmaceutics classification system. Int J Pharm. 2006;321:1–11.
Williams HD, Trevaskis NL, Charman SA, Shanker RM, Charman WN, Pouton CW, et al. Strategies to address low drug solubility in discovery and development. Pharmacol Rev. 2013;65:315–499.
Sugano K. A simulation of oral absorption using classical nucleation theory. Int J Pharm. 2009;378:142–5.
Huang Y, Dai WG. Fundamental aspects of solid dispersion technology for poorly soluble drugs. Acta Pharm Sin B. 2014;4:18–25.
Alonzo DE, Gao Y, Zhou D, Mo H, Zhang GGZ, Taylor LS. Dissolution and precipitation behavior of amorphous solid dispersions. J Pharm Sci. 2011;100:3316–31.
Alonzo DE, Zhang GG, Zhou D, Gao Y, Taylor LS. Understanding the behavior of amorphous pharmaceutical systems during dissolution. Pharm Res. 2010;27:608–18.
Wang C, Perumalla SR, Lu R, Fang J, Berberine CCSS. Sweet Berberine. Cryst Growth Des. 2016;16:933–9.
Avdeef A, Tsinman O. Miniaturized rotating disk intrinsic dissolution rate measurement: effects of buffer capacity in comparisons to traditional Wood's apparatus. Pharm Res-Dordr. 2008;25:2613–27.
Wang CG, Hu SY, Sun CQC. Expedited development of diphenhydramine orally disintegrating tablet through integrated crystal and particle engineering. Mol Pharm. 2017;14:3399–408.
Rodriguez-Hornedo N, Nehru SJ, Seefeldt KF, Pagan-Torres Y, Falkiewicz CJ. Reaction crystallization of pharmaceutical molecular complexes. Mol Pharm. 2006;3:362–7.
Harris RK, Ghi PY, Puschmann H, Apperley DC, Griesser UJ, Hammond RB, et al. Structural studies of the polymorphs of carbamazepine, its dihydrate, and two solvates. Org Process Res Dev. 2005;9:902–10.
Ilevbare GA, Liu HY, Edgar KJ, Taylor LS. Maintaining supersaturation in aqueous drug solutions: impact of different polymers on induction times. Cryst Growth Des. 2013;13:740–51.
Wang C, Perumalla SR, Lu R, Fang J, Sun CC. Sweet Berberine. Cryst Growth Des. 2016;16:933–9.
Chow SF, Shi L, Ng WW, Leung KHY, Nagapudi K, Sun CC, et al. Kinetic entrapment of a hidden curcumin Cocrystal with Phloroglucinol. Cryst Growth Des. 2014;14:5079–89.
Hou H, CC Sun. Quantifying effects of particulate properties on powder flow properties using a ring shear tester. J Pharm Sci. 2008;97:4030–9.
Sun CC. Quantifying effects of moisture content on flow properties of microcrystalline cellulose using a ring shear tester. Powder Technol. 2016;289:104–8.
Zhou Q, Shi L, Chattoraj S, Sun CC. Preparation and characterization of surface-engineered coarse microcrystalline cellulose through dry coating with silica nanoparticles. J Pharm Sci. 2012;101:4258–66.
Porter WW, Elie SC, Matzger AJ. Polymorphism in carbamazepine cocrystals. Cryst Growth Des. 2008;8:14–6.
Buanz ABM, Parkinson GN, Gaisford S. Characterization of carbamazepine-Nicatinamide Cocrystal polymorphs with rapid heating DSC and XRPD. Cryst Growth Des. 2011;11:1177–81.
Murphy D, Rodriguez-Cintron F, Langevin B, Kelly RC, Rodriguez-Hornedo N. Solution-mediated phase transformation of anhydrous to dihydrate carbamazepine and the effect of lattice disorder. Int J Pharm. 2002;246:121–34.
Gao P, Shi Y. Characterization of Supersaturatable formulations for improved absorption of poorly soluble drugs. AAPS J. 2012;14:703–13.
Joiris E, Di Martino P, Berneron C, Guyot-Hermann AM, Guyot JC. Compression behavior of orthorhombic paracetamol. Pharm Res. 1998;15:1122–30.
Sun CC, Hou H, Gao P, Ma C, Medina C, Alvarez FJ. Development of a high drug load tablet formulation based on assessment of powder manufacturability: moving towards quality by design. J Pharm Sci. 2009;98:239–47.
Sun CC. Setting the bar for powder flow properties in successful high speed tableting. Powder Technol. 2010;201:106–8.
Shi L, Feng YS, Sun CC. Origin of profound changes in powder properties during wetting and nucleation stages of high-shear wet granulation of microcrystalline cellulose. Powder Technol. 2011;208:663–8.
Carino SR, Sperry DC, Hawley M. Relative bioavailability estimation of carbamazepine crystal forms using an artificial stomach-duodenum model. J Pharm Sci-Us. 2006;95:116–25.
Ueda K, Higashi K, Yamamoto K, Moribe K. Inhibitory effect of hydroxypropyl methylcellulose acetate succinate on drug recrystallization from a supersaturated solution assessed using nuclear magnetic resonance measurements. Mol Pharm. 2013;10:3801–11.
Douroumis D, Bouropoulos N, Fahr A. Physicochemical characterization of solid dispersions of three antiepileptic drugs prepared by solvent evaporation method. J Pharm Pharmacol. 2007;59:645–53.
Rane Y, Mashru R, Sankalia M, Sankalia J. Effect of hydrophilic swellable polymers on dissolution enhancement of carbamazepine solid dispersions studied using response surface methodology. AAPS PharmSciTech. 2007;8:E1–E11.
Nair R, Gonen S, Hoag SW. Influence of polyethylene glycol and povidone on the polymorphic transformation and solubility of carbamazepine. Int J Pharm. 2002;240:11–22.
Li S, Wong S, Sethia S, Almoazen H, Joshi YM, Serajuddin AT. Investigation of solubility and dissolution of a free base and two different salt forms as a function of pH. Pharm Res. 2005;22:628–35.
Acknowledgments and Disclosures
Parts of this work were carried out in the Characterization Facility, University of Minnesota, a member of the NSF-funded Materials Research Facilities Network (http://www.mrfn.org) via the MRSEC program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 250 kb)
Rights and permissions
About this article
Cite this article
Yamashita, H., Sun, C.C. Expedited Tablet Formulation Development of a Highly Soluble Carbamazepine Cocrystal Enabled by Precipitation Inhibition in Diffusion Layer. Pharm Res 36, 90 (2019). https://doi.org/10.1007/s11095-019-2622-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-019-2622-7