Abstract
Key message
Role of Rubisco Activase in imparting thermotolerance to the photosynthetic apparatus under high temperature. Thus, to improve the grain filling, we need to fine tune these crucial enzymes and their regulation, which directly or indirectly affect spike photosynthesis.
Abstract
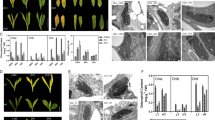
CO2 fixation in cereals crops like bread wheat (Triticum aestivum L.) is also contributed by ear photosynthesis beside the other organs like leaves or the flag leaf. 1000-grain weight of three Indian wheat cultivars (cvs.) PBW343, K7903, and HD2329 were calculated under three treatments until maturity stage (i.e. removal of flag leaf, removal of awns and shaded spikes). We observed that awn removal showed a significant decrease in 1000-grain weight in all cultivars. To delve deeper into the biological and molecular pathways taking place underlying the awn physiology, we conducted the awn transcriptome analysis of thermosusceptible Indian wheat cv. PBW343 under heat stress (HS) at 42 °C for 2 h using RNA-sequencing (RNA-seq). Differential expression analysis revealed, 160 transcripts, out of these, 143 transcripts were significantly upregulated and 17 transcripts were repressed under HS conditions. Of these Rca1β was selected for characterization and overexpression studies. Ectopic expression of TaRca1β in rice transgenics indicate a direct correlation with tolerance under HS conditions. TaRca1β provides a better photosynthate energy partitioning under HS with a significant reduction in the non-photochemical fluorescence quenching of the photosynthetic machinery.








Similar content being viewed by others
References
Abbad H, Jaafari SE, Bort J, Araus JL (2004) Agronomy for sustainable development. Ital J Agron 24:19–28
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Andersson I (2008) Catalysis and regulation in Rubisco. J Exp Bot 59:1555–1568
Andrews S (2010) FastQC a quality control tool for high throughput sequence data. https://www.bioinformatics.babraham.ac.uk/projects/fastqc.
Araus JL, Brown HR, Febrero A, Bort J, Serret MD (1993) Ear photosynthesis, carbon isotope discrimination and the contribution of respiratory CO2 to differences in grain mass in durum wheat. Plant Cell Environ 16:383–392
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM et al (2000) Gene ontology: tool for the unification of biology. Nat Genet 25:25–29
Blum A (1985) Photosynthesis and transpiration in leaves and ears of wheat and barley varieties. J Exp Bot 36:432–440
Bruening PW (2019) Effects of awns on wheat yield and agronomic characterstics evaluated in variety trials. J Crop Variety Test 2:1–5
Bruening B, Piersawl M, Swanson S, Connelley J, Olson G, Van Sanford D (2019) Performance of small grain varieties in kentcky. College of Ag, Food and Enviroment, Univ of Kentucky
Carmo-Silva AE, Salvucci ME (2013) The regulatory properties of Rubisco activase differ among species and affect photosynthetic induction during light transitions. Plant Physiol 161:1645–1655
Carmo-Silva E, Scales JC, Madgwick PJ, Parry MAJ (2015) Optimizing Rubisco and its regulation for greater resource use efficiency. Plant Cell Environ 38:1817–1832
Chauhan H, Khurana N, Tyagi AK, Khurana JP, Khurana P (2010) Identification and characterization of high temperature stress responsive genes in bread wheat (Triticum aestivum L.) and their regulation at various stages of development. Plant Mol Biol 75:35–51
Chen G, Bi YR, Li N (2005) EGY1 encodes a membrane-associated and ATP-independent metalloprotease that is required for chloroplast development. Plant J 41:364–375
Degen GE, Worrall D, Carmo-Silva E (2020) An isoleucine residue acts as a thermal and regulatory switch in wheat Rubisco activase. Plant J 103:742–751
Duwayri M (1984) Comparison of wheat cultivars grown in the field under different levels of moisture. Cereal Res Commun 12(1/2):27–34
Echevarría-Zomeño S, Fernández-Calvino L, Castro-Sanz AB, López JA, Vázquez J, Castellano MM (2016) Dissecting the proteome dynamics of the early heat stress response leading to plant survival or death in Arabidopsis. Plant Cell Environ 39:1264–1278
Evans LT, Bingham J, Jackson P, Sutherland J (1972) Effect of awns and drought on the supply of photosynthate and its distribution within wheat ears. Ann Appl Biol 70:67–76
Evans LT, Wardlaw IF, Fisher RA (1975) Crop physiology some case histories. The University of Chicago, Cambridge, UK
Garrido J, Aguilar M, Prieto P (2020) Identification and validation of reference genes for RT-qPCR normalization in wheat meiosis. Sci Rep 10:2726
Gebbing T, Schnyder H (1999) Pre-anthesis reserve utilization for protein and carbohydrate synthesis in grains of wheat. Plant Physiol 121:871–878
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. Biochem Biophys Acta 990:87–92
Giunta F, Motzo R, Deidda M (2002) SPAD readings and associated leaf traits in durum wheat, barley and triticale cultivars. Euphytica 125:197–205
Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I et al (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29:644–652
Grundbacher FJ (1963) The physiological function of the cereal awn. Bot Rev 29:366–381
Haas BJ, Papanicolao A, Yassour M, Grabherr M, Blood PD, Bowden J et al (2013) De novo transcript sequence reconstruction from RNA-seq using the trinity platform for reference generation and analysis. Nat Protoc 8:1494–1512
Himmelbach A, Zierold U, Hensel G, Riechen J, Douchkov D, Schweizer P et al (2007) A set of modular binary vectors for transformation of cereals. Plant Physiol 145:1192–1200
Kabbage M, Dickman MB (2008) The BAG proteins: a ubiquitous family of chaperone regulators. Cell Mol Life Sci 65:1390–1402
Kumar RR, Rai RD (2014) Can wheat beat the heat: understanding the mechanism of thermotolerance in wheat (Triticum aestivum L.). Cereal Res Commun 42:1–18
Kumar A, Li C, Portis AR (2009) Arabidopsis thaliana expressing a thermostable chimeric Rubisco activase exhibits enhanced growth and higher rates of photosynthesis at moderately high temperatures. Photosynth Res 100:143–153
Kurek I, Chang TK, Bertain SM, Madrigal A, Liu L, Lassner MW et al (2007) Enhanced thermostability of Arabidopsis Rubisco activase improves photosynthesis and growth rates under moderate heat stress. Plant Cell 19:3230–3241
Langmead B, Trapnell C, Pop M et al (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. https://doi.org/10.1186/gb-2009-10-3-r25
Law RD, Crafts-Brandner SJ (2001) High temperature stress increases the expression of wheat leaf ribulose-1,5-bisphosphate carboxylase/oxygenase activase protein. Arch Biochem Biophys 386:261–267
Li X, Wang H, Li H, Zhang L, Teng N, Lin Q et al (2006) Awns play a dominant role in carbohydrate production during the grain-filling stages in wheat (Triticum aestivum). Physiol Plant 127:701–709
Lorimer GH (1981) The carboxylation and events in photosynthesis and photorespiration. Annu Rev Plant Physiol 32:349–383
Lorimer GH, Miziorko HM (1980) Carbamate formation on the epsilon-amino group of a lysyl residue as the basis for the activation of ribulosebisphosphate carboxylase by CO2 and Mg2+. Biochemistry 19(23):5321–5328
Miller EC, Gauch HG, Gries GA (1944) A study of the morpho- logical nature and physiological function of the awns in winter wheat. Agricultural Experiment Station, Kansas
McMaster GS, Wilhelm WW (1997) Growing degree-days: one equation, two interpretations. Agric For Meteorol 87:291–300
Murchie EH, Lawson T (2013) Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. J Exp Bot 64:3983–3998
Neuwald AF, Aravind L, Spouge JL, Koonin EV (1999) AAA+: a class of chaperone-like atpases associated with the assembly, operation, and disassembly of protein complexes. Genome Res 9:27–43
Neuwirth E (2007) RColorBrewer: ColorBrewer palettes. R package version 11:0–2
Nishimura A, Aichi I, Matsuoka M (2007) A protocol for Agrobacterium-mediated transformation in rice. Nat Protoc 1:2796–2802
Portis AR (1990) Rubisco activase. Biochim Biophys Acta 1015:15–28
Paolacci AR, Tanzarella OA, Porceddu E et al (2009) Identification and validation of reference genes for quantitative RT–PCR normalization in wheat. BMC Molecular Biol 10:11
Ristic Z, Momilović I, Bukovnik U, Prasad PVV, Fu J, Deridder BP et al (2009) Rubisco activase and wheat productivity under heat-stress conditions. J Exp Bot 60:4003–4014
Robinson MD, McCarthy DJ, Smyth GK (2009) edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26:139–140
Saghir AR, Khan AR, Worzella WW (1968) Effects of plant parts on the grain yield, kernel weight, and plant height of wheat and barley1. Agron J 60:95–97
Salvucci ME, Ogren WL (1996) The mechanism of Rubisco activase: insights from studies of the properties and structure of the enzyme. Photosynth Res 47:1–11
Sanchez-bragado R, Molero G, Reynolds MP, Araus JL (2014) Relative contribution of shoot and ear photosynthesis to grain filling in wheat under good agronomical conditions assessed by differential organ δ13 C. J Exp Bot 65:5401–5413
Scharen AL, Krupinsky JM, Reid DA (1983) Photosynthesis and yield of awned versus awnless isogenic lines of winter barley. Can J Plant Sci 63:349–355
Schaller CW, Qualset CO (1975) Isogenic analysis of productivity in Barley: Interaction of genes affecting awn length and leaf-spotting1. Crop Sci 15:378–382
Spreitzer RJ, Salvucci ME (2002) Rubisco: structure, regulatory interactions, and possibilities for a better enzyme. Annu Rev Plant Biol 53:449–475
Stotz M, Mueller-Cajar O, Ciniawsky S, Wendler P, Hartl FU, Bracher A et al (2011) Structure of green-type Rubisco activase from tobacco. Nat Struct Mol Biol 18:1366–1370
Tambussi EA, Bort J, Guiamet JJ, Nogués S, Araus JL (2007) The photosynthetic role of ears in C3 cereals: metabolism, water use efficiency and contribution to grain yield. Crit Rev Plant Sci 26:1–16
Toki S, Hara N, Ono K, Onodera H, Tagiri A, Oka S et al (2006) Early infection of scutellum tissue with Agrobacterium allows high-speed transformation of rice. Plant J 47:969–976
Warnes GR, Bolker B, Bonebakker L, Gentleman R, Huber W, Liaw WHA et al (2009) gplots: various R programming tools for plotting data. CRAN 2(4):1
Werneke JM, Chatfield JM, Ogren WL (1988) Catalysis of ribulosebisphosphate carboxylase/oxygenase activation by the product of a Rubisco activase cdna clone expressed in Escherichia coli. Plant Physiol 87:917–920
Zadoks JC, Board E (1974) A decimal code for the growth stages of cereals. Weed Res 14:415–421
Acknowledgements
Authors are thankful to Department of Biotechnology and Department of Science &Technology-Fund for Improvement of S&T Infrastructure (DST-FIST), Government of India (Grant No. BT/PR9416/AGR/02/412/2007) for the financial support. We also thanks Dr. Jochen Kumlehn, Leibniz Institute of Plant Genetic and Crop Plant Research (IPK) Gatersleben for providing the IPKb vector set.
Author information
Authors and Affiliations
Contributions
CK, NS: performed the experiments. CC, PK: written and formatted the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they do not have any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chaudhary, C., Sharma, N. & Khurana, P. Decoding the wheat awn transcriptome and overexpressing TaRca1β in rice for heat stress tolerance. Plant Mol Biol 105, 133–146 (2021). https://doi.org/10.1007/s11103-020-01073-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-020-01073-0