Abstract
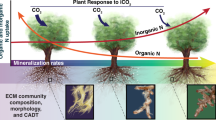
Many forest trees have evolved mutualistic symbioses with ectomycorrhizal (ECM) fungi that contribute to their phosphorus (P) nutrition. Forest productivity is frequently limited by P, a phenomenon that is likely to become more widespread under future conditions of elevated atmospheric CO2 concentration [CO2]. It is thus timely that this review considers current understanding of the key processes (absorption, translocation and transfer to the plant host) in ECM fungus-mediated P nutrition of forest trees. Solubilisation of inorganic P (Pi) and hydrolysis of organic P by ECM fungi in soil occurs largely at the growing mycelial front, where Pi absorption is facilitated by high affinity transporters. While large gaps remain in our understanding of the physiological and molecular mechanisms that underpin movement of P in ECM mycelia in soil and P transfer to the plant, host P demand seems likely to be a key driver of these processes. ECM fungi may make considerable contributions to meeting the likely increased P demand of trees under elevated [CO2] via increased colonization levels, shifts in ECM fungal community structure and changed patterns of EMM production. Further research into the spatial scale of ECM-mediated P movements in soil, along with the interplay between ECM fungi and other soil microflora is advocated.

Similar content being viewed by others
References
Agerer R (2001) Exploration types of ectomycorrhizae. Mycorrhiza 11:107–114
Ahonen Jonnarth U, van Hees PAW, Lundström US, Finlay RD (2000) Organic acids produced by mycorrhizal Pinus sylvestris exposed to elevated aluminium and heavy metal concentrations. New Phytol 146:557–567
Alexander IJ, Hardy K (1981) Surface phosphatase activity of Sitka spruce mycorrhizas from serpentine soil. Soil Biol Biochem 13:301–305
Ali MA, Louche J, Legname E, Duchemin M, Plassard C (2009) Pinus pinaster seedlings and their fungal symbionts show high plasticity in phosphorus acquisition in acidic soils. Tree Physiol 29:1587–1597
Allaway WG, Ashford AE (2001) Motile tubular vacuoles in extramatrical mycelium and sheath hyphae of ectomycorrhizal systems. Protoplasma 215:218–225
Alvarez M, Godoy R, Heyser W, Härtel S (2004) Surface-bound phosphatase activity in living hyphae of ectomycorrhizal fungi of Nothofagus obliqua. Mycologia 96:479–487
Alvarez M, Godoy R, Heyser W, Härtel S (2005) Anatomical-physiological determination of surface bound phosphatase activity in ectomycorrhizae of Nothofagus obliqua. Soil Biol Biochem 37:125–132
Alvarez M, Gieseke A, Godoy R, Härtel S (2006) Surface-bound phosphatase activity in ectomycorrhizal fungi: a comparative study between a colorimetric and microscope-based method. Biol Fertil Soils 42:561–568
Alvez L, Oliveira VL, Filho GNS (2010) Utilization of rocks and ectomycorrhizal fungi to promote growth of eucalypt. Braz J Microbiol 41:676–684
Anderson IC, Cairney JWG (2007) Ectomycorrhizal fungi: exploring the mycelial frontier. FEMS Microbiol Rev 31:388–406
Antibus SK, Croxdale JG, Miller OK, Linkins AE (1981) Ectomycorrhizal fungi of Salix rotundifolia III. Resynthesised mycorrhizal complexes and their surface phosphatase activities. Can J Bot 59:2458–2465
Antibus RK, Kroehler CJ, Linkins AE (1986) The effects of external pH, temperature, and substrate concentration on acid phosphatase activity of ectomycorrhizal fungi. Can J Bot 64:2383–2387
Antibus RK, Sinsabaugh RL, Linkins AE (1992) Phosphatase activities and phosphorus uptake from inositol phosphate by ectomycorrhizal fungi. Can J Bot 70:794–801
Antibus RK, Bower D, Dighton J (1997) Root surface phosphatase activities and uptake of 32P-labelled inositol phosphate in field-collected gray birch and red maple roots. Mycorrhiza 7:39–46
Arvieu J-C, Leprince F, Plassard C (2003) Release of oxalate and protons by ectomycorrhizal fungi in response to P-deficiency and calcium carbonate in nutrient solution. Ann For Sci 60:815–821
Ashford AE (1998) Dynamic pleiomorphic vacuole systems: are they endosomes and transport compartments in fungal hyphae? Adv Bot Res 28:119–159
Ashford AE, Allaway WG (2002) The role of the motile tubular vacuole system in mycorrhizal fungi. Plant Soil 244:177–187
Ashford AE, Allaway WG, Peterson CA, Cairney JWG (1989) Nutrient transfer and the fungus-root interface. Aust J Plant Physiol 16:85–97
Attiwill PM, Leeper GW (1987) Forest soils and nutrient cycles. Melbourne University Press, Melbourne
Baghel RK, Sharma R, Pandey AK (2009) Activity of acid phosphatase in the ectomycorrhizal fungus Cantharellus tropicalis under controlled conditions. J Trop For Sci 21:218–222
Bakker MR, Jolicoeur E, Trichet P, Augusto L, Plassard C, Guinberteau J, Loustau D (2009) Adaptation of fine roots to annual fertilization and irrigation in a 13-year-old Pinus pinaster stand. Tree Physiol 29:229–238
Bartlett EM, Lewis DH (1973) Surface phosphatase activity of mycorrhizal roots of beech. Soil Biol Biochem 5:249–257
Baxter JW, Dighton J (2001) Ectomycorrhizal diversity alters growth and nutrient acquisition of grey birch (Betula populifolia) seedlings in host-symbiont culture conditions. New Phytol 152:139–149
Baxter JW, Dighton J (2005) Phosphorus source alters host plant response to ectomycorrhizal diversity. Mycorrhiza 15:513–523
Beever RE, Burns DJW (1981) Phosphorus uptake, storage and utilization by fungi. Adv Bot Res 8:127–219
Bending GD, Read DJ (1995) The structure and function of the vegetative mycelium of ectomycorrhizal plants. V. Foraging behaviour and translocation of nutrients from exploited organic matter. New Phytol 130:401–409
Bernston GM, Wayne PM, Bazzaz FA (1997) Below-ground architectural and mycorrhizal responses to elevated CO2 in Betula alleghaniensis populations. Funct Ecol 11:684–695
Bidartondo MI, Ek H, Wallander H, Söderström B (2001) Do nutrient additions alter carbon sink strength of ectomycorrhizal fungi? New Phytol 151:543–550
Bieleski RL (1973) Phosphate pools, phosphate transport and phosphate availability. Adv Bot Res 24:225–252
Blum LD, Klaue A, Nezat CA, Driscoll CT, Johnson CE, Siccama TG, Eagar C, Fahey TJ, Likens GE (2002) Mycorrhizal weathering of apatite as an important calcium source in base-poor forest ecosystems. Nature 417:729–731
Bolan NS (1991) A critical review on the role of mycorrhizal fungi in the uptake of phosphorus by plants. Plant Soil 134:189–207
Bonello P, Bruns TD, Gardes M (1998) Genetic structure of a natural population of the ectomycorrhizal fungus Suillus pungens. New Phytol 138:533–542
Bougher NL, Grove TS, Malajczuk N (1990) Growth and phosphorus acquisition of karri (Eucalyptus diversicolor F. Muell.) seedlings inoculated with ectomycorrhizal fungi in relation to phosphorus supply. New Phytol 114:77–85
Brandes B, Godbold DL, Kuhn AJ, Jentschke G (1998) Nitrogen and phosphorus acquisition by the mycelium of the ectomycorrhizal fungus Paxillus involutus and its effect on host nutrition. New Phytol 140:735–743
Bucher M (2007) Functional biology of plant phosphate uptake at root and mycorrhiza interfaces. New Phytol 173:11–26
Bücking H (2004) Phosphate absorption and efflux of three ectomycorrhizal fungi as affected by external phosphate, cation and carbohydrate concentrations. Mycol Res 108:599–609
Bücking H, Heyser W (1999) Elemental composition and function of polyphosphates in ectomycorrhizal fungi—an X-ray microanalysis study. Mycol Res 103:31–39
Bücking H, Heyser W (2000) Subcellular compartmentation of elements in non-mycorrhizal and mycorrhizal roots of Pinus sylvestris: an X-ray microanalytical study. I. The distribution of phosphate. New Phytol 145:311–320
Bücking H, Heyser W (2001) Microrautoradiographic localization of phosphate and carbohydrate in mycorrhizal roots of Populus tremela x Populus alba and the implications for transfer processes in ectomycorrhizal associations. Tree Physiol 21:101–107
Buée M, Vairelles D, Garbaye J (2005) Year-round monitoring of diversity and potential metabolic activity of the ectomycorrhizal community in a beech (Fagus sylvatica) forest subjected to two thinning regimes. Mycorrhiza 15:235–245
Buscot F, Munch JC, Charcosset JY, Gardes M, Nehls HR (2000) Recent advances in exploring physiology and biodiversity of ectomycorrhizas highlight the functioning of these symbioses in ecosystems. FEMS Microbiol Rev 24:601–614
Cairney JWG (1992) Translocation of solutes in ectomycorrhizal and saprotrophic rhizomorphs. Mycol Res 96:135–141
Cairney JWG (1999) Intraspecific physiological variation: implications for understanding functional diversity in ectomycorrhizal fungi. Mycorrhiza 9:125–135
Cairney JWG (2005) Basidiomycete mycelia in forest soils: dimensions, dynamics and roles in nutrient distribution. Mycol Res 109:7–20
Cairney JWG, Alexander IJ (1992a) A study of ageing of spruce [Picea sitchensis (Bong.) Carr.] ectomycorrhizas. II. Carbon allocation in ageing Picea sitchensis/Tylospora fibrillosa (Burt.) Donk ectomycorrhizas. New Phytol 122:153–158
Cairney JWG, Alexander IJ (1992b) A study of ageing of spruce [Picea sitchensis (Bong.) Carr.] ectomycorrhizas. III. Phosphate absorption and transfer in ageing Picea sitchensis/Tylospora fibrillosa (Burt.) Donk ectomycorrhizas. New Phytol 122:159–164
Cairney JWG, Burke RM (1994) Fungal enzymes degrading plant cell walls: their possible significance in the ectomycorrhizal symbiosis. Mycol Res 98:1345–1356
Cairney JWG, Burke RM (1996) Physiological heterogeneity within fungal mycelia: an important concept for a functional understanding of the ectomycorrhizal symbiosis. New Phytol 134:685–695
Cairney JWG, Smith SE (1992) Influence of intracellular phosphorus concentration on phosphate absorption by the ectomycorrhizal basidiomycete Pisolithus tinctorius. Mycol Res 96:673–676
Cairney JWG, Smith SE (1993a) Efflux of phosphate from the ectomycorrhizal basidiomycete Pisolithus tinctorius: general characteristics and the influence of intracellular phosphorus concentration. Mycol Res 97:1261–1266
Cairney JWG, Smith SE (1993b) The influence of monovalent cations on efflux of phosphate from the ectomycorrhizal basidiomycete Pisolithus tinctorius. Mycol Res 97:1267–1271
Cairney JWG, Jennings DH, Agerer R (1991) The nomenclature of fungal multi-hyphal linear aggregates. Crypt Bot 2(3):246–251
Calvaruso C, Turpault M-P, Leclerc E, Frey-Klett P (2007) Impact of ectomycorrhizosphere on the functional diversity of soil bacterial and fungal communities from a forest stand in relation to nutrient mobilization processes. Microb Ecol 54:567–577
Caserin V, Plassard C, Souche G, Arvieu J-C (2003) Quantification of oxalate ions and protons released by ectomycorrhizal fungi in rhizosphere soil. Agronomie 23:461–469
Caserin V, Plassard C, Hinsinger P, Arvieu J-C (2004) Quantification of ectomycorrhizal fungal effects on the bioavailability and mobilization of soil P in the rhizosphere of Pinus pinaster. New Phytol 163:177–185
Chen CR, Condron LM, Xu ZH (2008) Impacts of grassland afforestation with coniferous trees on soil phosphorus dynamics and associated processes: a review. For Ecol Manag 255:396–409
Choi DS, Quoreshi AM, Maruyama Y, Jin HO, Koike T (2005) Effect of ectomycorrhizal infection on growth and photosynthesis characteristics of Pinus densiflora seedlings grown under elevated CO2 concentrations. Photosynthetica 43:223–229
Clarkson DT (1985) Factors affecting mineral nutrient acquisition by plants. Ann Rev Plant Physiol 36:77–115
Clipson NJW, Cairney JWG, Jennings DH (1987) The physiology of basidiomycete linear organs. I. Phosphate uptake by cords and mycelium in the laboratory and the field. New Phytol 105:449–457
Colpaert JV, Verstuyft I (1999) The Ingestad concept in ectomycorrhizal research: possibilities and limitations. Physiol Plant 105:233–238
Colpaert JV, Van Laere A, Van Tichelen KK, Van Assche JA (1997) The use of inositol hexaphosphate as a phosphorus source by mycorrhizal and non-mycorrhizal Scots pine (Pinus sylvestris). Funct Ecol 11:407–415
Colpaert JV, Van Tichelen KK, Van Assche JA, Van Laere A (1999) Short-term phosphorus uptake rates in mycorrhizal and non-mycorrhizal roots of intact Pinus sylvestris seedlings. New Phytol 143:589–597
Conn C, Dighton J (2000) Litter quality influences on decomposition, ectomycorrhizal community structure and mycorrhizal root surface acid phosphatase activity. Soil Biol Biochem 32:489–496
Conroy JP, Küppers M, Küppers B, Vigona J, Barlow EWR (1988) The influence of CO2 enrichment, phosphorus deficiency and water stress on the growth, conductance and water use of Pinus radiata D. Don. Plant Cell Environ 11:91–98
Conroy JP, Milham PJ, Reed ML, Barlow EWR (1990) Increases in phosphorus requirements for CO2 enriched pine species. Plant Physiol 92:977–982
Conroy JP, Milham PG, Barlow EWR (1992) Effects of nitrogen and phosphorus availability on the growth response of Eucalyptus grandis to high CO2. Plant Cell Environ 15:843–847
Courty P-E, Pouysegur R, Buée M, Garbaye J (2006) Laccase and phosphatase activities of the dominant ectomycorrhizal types in a lowland oak forest. Soil Biol Biochem 38:1219–1222
Courty P-E, Bréda N, Garbaye J (2007) Relation between oak tree phenology and the secretion of organic matter degrading enzymes by Lactarius quietus ectomycorrhizas before and during bud break. Soil Biol Biochem 39:1655–1663
Cromack K, Sollins P, Graustein WC, Speidel K, Todd AW, Spycher G, Li CY, Todd RL (1979) Calcium oxalate accumulation and soil weathering in mats of the hypogeous fungus Hysterangium crassum. Soil Biol Biochem 11:463–468
Cullings K, Ishkhanova G, Ishkhanov G, Henson G (2010) Induction of saprophytic behaviour in the ectomycorrhizal fungus Suillus granulatus by litter addition in a Pinus contorta (Lodgepole pine) stand in Yellowstone. Soil Biol Biochem 42:1176–1178
Cumming JR (1993) Growth and nutrition of nonmycorrhizal and mycorrhizal pitch pine (Pinus rigida) seedlings under phosphorus limitation. Tree Physiol 13:173–187
Cumming JR (1996) Phosphate-limitation physiology in ectomycorrhizal pitch pine (Pinus rigida) seedlings. Tree Physiol 16:977–983
Cumming JR (1998) Integrated phosphorus acquisition strategies in ectomycorrhizal fungi. In: Lynch JP, Deikman J (eds) Phosphorus in plant biology: regulatory roles in molecular, cellular, organismic, and ecosystem processes. American Society of Plant Physiologists, Rockville, pp 124–135
Dahlberg A, Stenlid J (1990) Population structure and dynamics in Suillus bovinus as indicated by spatial distribution of fungal clones. New Phytol 115:487–493
Dalal RC (1977) Soil organic phosphorus. Adv Agron 29:83–117
Darrah PR, Tlalka M, Ashford A, Watkinson SC, Fricker MD (2006) The vacuole system is a significant intracellular pathway for longitudinal solute transport in basidiomycete fungi. Eucaryotic Cell 5:1111–1125
de la Bastide PY, Kropp BR, Piché Y (1995) Population structure and mycelial phenotypic variability of the ectomycorrhizal basidiomycete Laccaria bicolor (Maire) Orton. Mycorrhiza 5:371–379
DeLucia EH, Callaway RM, Thomas EM, Schlesinger WH (1997) Mechanisms of phosphorus acquisition for ponderosa pine seedlings under high CO2 and temperature. Ann Bot 79:111–120
Di HJ, Condron LM, Frossard E (1997) Isotope techniques to study phosphorus cycling in agricultural and forest soils: a review. Biol Fertil Soils 24:1–12
Dickie IA, Richardson SJ, Wiser SK (2009) Ectomycorrhizal fungal communities and soil chemistry in harvested and unharvested temperate Nothofagus rainforests. Can J For Res 39:1069–1079
Dighton J (1983) Phosphatase production by mycorrhizal fungi. Plant Soil 71:455–462
Dighton J, Harrison AF (1990) Changes in phosphate status of Sitka-spruce plantations of increasing age, as determined by root bioassay. For Ecol Manag 31:35–44
Dighton J, Mason PA, Poskitt JM (1990) Field use of 32P to measure phosphate uptake by birch mycorrhizas. New Phytol 116:655–661
Dighton J, Poskitt JM, Brown TK (1993) Phosphate influx into ectomycorrhizal and saprotrophic fungal hyphae in relation to phosphate supply; a potential method for selection of efficient mycorrhizal species. Mycol Res 97:355–358
Dominguez Núñez JAD, Serrano JS, Barreal JAR, Gonzales JASD (2006) The influence of mycorrhization with Tuber melanosporum in the afforestation of a Mediterranean site with Quercus ilex and Quercus faginea. For Ecol Manag 231:226–233
Doumas P, Berjaud C, Calléja M, Coupé M, Espiau C, d’Auzac J (1986) Phosphatases extracellulaires et nutrition phosphatée les champignons ectomycorrhiziens et les plantes hôtes. Physiol Veg 24:173–184
Dunham SM, Kretzer A, Pfrender ME (2003) Characterization of Pacific golden chanterelle (Cantharellus formosus) genet size using co-dominant microsatellite markers. Mol Ecol 12:1607–1618
Ekblad A, Wallander H, Carlsson R, Huss-Danell K (1995) Fungal biomass in roots and extramatrical mycelium in relation to macronutrients and plant biomass of ectomycorrhizal Pinus sylvestris and Alnus incana. New Phytol 131:443–451
Ezawa T, Smith SE, Smith FA (2001) Differentiation of polyphosphate metabolism between the extra- and intraradical hyphae of arbuscular mycorrhizal fungi. New Phytol 149:555–563
Finlay RD (1993) Uptake and mycelial translocation of nutrients by ectomycorrhizal fungi. In: Read DJ, Lewis DH, Fitter AH, Alexander IJ (eds) Mycorrhizas in ecosystems. CAB International, Wallingford, pp 91–97
Finlay RD, Read DJ (1986) The structure and function of the vegetative mycelium of ectomycorrhizal plants. II. The uptake and distribution of phosphorus by mycelial strands interconnecting host plants. New Phytol 103:157–165
Fomina M, Alexander IJ, Hillier S, Gadd GM (2004) Zinc phosphate and pyromorphite solubilisation by soil plant-symbiotic fungi. Geomicrobiol J 21:351–366
Fomina M, Charnock JM, Hillier S, Alexander IJ, Gadd GM (2006) Zinc phosphate transformations by the Paxillus involutus/pine ectomycorrhizal association. Microb Ecol 52:322–333
Fransson PMA, Taylor AFS, Finlay RD (2001) Elevated atmospheric CO2 alters root symbiont community structure in forest trees. New Phytol 152:431–442
Fransson PMA, Taylor AFS, Finlay RD (2005) Mycelial production, spread and root colonisation by the ectomycorrhizal fungi Hebeloma crustuliniforme and Paxillus involutus under elevated atmospheric CO2. Mycorrhiza 15:25–31
Frey-Klett P, Chavatte M, Clausse M-L, Courrier S, Le Roux C, Raaijmakers J, Martinotti MG, Pierrat J-C, Garbaye J (2005) Ectomycorrhizal symbiosis affects functional diversity of rhizosphere fluorescent pseudomonads. New Phytol 165:317–328
Fricker MN, Lee JA, Bebber DP, Tlalka M, Hynes J, Darrah PR, Watkinson SC, Boddy L (2008) Imaging complex nutrient dynamics in mycelial networks. J Microsc 231:317–331
Garcia MO, Ovasapyan T, Greas M, Treseder KK (2008) Mycorrhizal dynamics under elevated CO2 and nitrogen fertilization in a warm temperate forest. Plant Soil 303:301–310
Genney DR, Anderson IC, Alexander IJ (2006) Fine-scale distribution of pine ectomycorrhizas and their extramatrical mycelium. New Phytol 170:381–390
Gharieb MM, Gadd GM (1999) Influence of nitrogen source on the solubilization of natural gypsum and the formation of calcium oxalate by different oxalic and citric acid-producing fungi. Mycol Res 103:473–481
Godbold DL, Berntson GM (1997) Elevated atmospheric CO2 concentration changes ectomycorrhizal morphotype assemblages in Betula papyrifera. Tree Physiol 17:347–350
Godbold DL, Bernston GM, Bazzaz FA (1997) Growth and mycorrhizal colonization of three North American tree species under elevated atmospheric CO2. New Phytol 137:433–440
Gradowski T, Thomas SC (2006) Phosphorus limitation of sugar maple growth in central Ontario. For Ecol Manag 226:104–109
Grierson PF, Commerford NB (2000) Non-destructive measurement of acid phosphatase activity in the rhizosphere using nitrocellulose membranes and image analysis. Plant Soil 218:49–57
Griffiths RP, Caldwell BA (1992) Mycorrhizal mat communities in forest soils. In: Read DJ, Lewis DH, Fitter AH, Alexander IJ (eds) Mycorrhizas in ecosystems. CAB International, Wallingford, pp 98–105
Griffiths RP, Baham JE, Caldwell BA (1994) Soil solution chemistry of ectomycorrhizal mats in forest soil. Soil Biol Biochem 26:331–337
Gryta H, Debaud J-C, Effosse A, Gay G, Marmeisse R (1997) Fine-scale structure of polulations of the ectomycorrhizal fungus Hebeloma cylindrosporum in coastal sand dune forest ecosystems. Mol Ecol 6:353–364
Hagerberg D, Thelin G, Wallander H (2003) The production of ectomycorrhizal mycelium in forests: relation between forest nutrient status and local mineral sources. Plant Soil 252:279–290
Harley JL, McCready CC (1952) The uptake of phosphate by excised mycorrhizal roots of the beech. II. Distribution of phosphorus between host and fungus. New Phytol 51:56–64
Harley JL, McCready CC (1981) Phosphate accumulation in Fagus mycorrhizas. New Phytol 89:75–80
Harrington TJ, Mitchell DT (2005) Ectomycorrhizas associated with a relict population of Dryas octopetala in the Burren, western Ireland. I. Distribution of ectomycorrhizas in relation to vegetation and soil characteristics. Mycorrhiza 15:425–433
Heinrich PA, Patrick JW (1986) Phosphorus acquisition in the soil-root system of Eucalyptus pilularis Smith seedlings. II. The effect of ectomycorrhizas on seedling phosphorus and dry weight acquisition. Aust J Bot 34:445–454
Hijikata N, Murase M, Tani C, Ohtomo R, Osaki M, Ezawa T (2010) Polyphosphate has a central role in the rapid and massive accumulation of phosphorus in extra radical mycelium of an arbuscular mycorrhizal fungus. New Phytol 186:285–289
Hinsinger P (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 226:275–295
Hinsinger P, Plassard C, Tang C, Jaillard B (2003) Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints—a review. Plant Soil 248:43–59
Hitchcock CJ, Chambers SM, Cairney JWG (2011) Genetic population structure of the ectomycorrhizal fungus Pisolithus microcarpus suggests high gene flow in south-eastern Australia. Mycorrhiza 21:131–137
Ho I (1987) Comparison of eight Pisolithus tinctorius isolates for growth rate, enzyme activity, and phytohormone production. Can J For Res 17:31–53
Hrynkiewicz K, Baum C, Leinweber P (2009) Mycorrhizal community structure, microbial biomass P and phosphatase activities under Salix polaris as influenced by nutrient availability. Eur J Soil Biol 45:168–175
Ineichen K, Wiemken V, Wiemken A (1995) Shoots, roots and ectomycorrhiza formation of pine seedlings at elevated atmospheric carbon dioxide. Plant Cell Environ 18:703–707
Javot H, Penmetsa RV, Terzaghi N, Cook DR, Harrison MJ (2007a) A Medicago truncatula phosphate transporter indispensible for the arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci USA 104:1720–1725
Javot H, Pumplin N, Harrison MJ (2007b) Phosphate in the arbuscular mycorrhizal symbiosis: transport properties and regulatory roles. Plant Cell Environ 30:310–322
Jayakumar P, Tan TK (2005) Phosphorus solubilisation by ectomycorhhizal Pisolithus tinctorius in pure culture and in association with Acacia mangium. Symbiosis 39:125–130
Jentschke G, Brandes B, Kuhn AJ, Schröder WH, Godbold DL (2001) Interdependence of phosphorus, nitrogen, potassium and magnesium translocation by the ectomycorrhizal fungus Paxillus involutus. New Phytol 149:327–337
Johansson EM, Fransson PMA, Finlay RD, van Hees PAW (2009) Quantitative analysis of soluble exudates produced by ectomycorrhizal roots as a response to ambient and elevated CO2. Soil Biol Biochem 41:1111–1116
Johnson DW, Hungate BA, Dijkstra P, Hymus G, Hinkle CR, Stiling P, Drake BG (2003) The effects of elevated CO2 on nutrient distribution in a fire-adapted scrub oak forest. Ecol Appl 13:1388–1399
Jones DL (1998) Organic acids in the rhizosphere—a critical review. Plant Soil 205:25–44
Jones MD, Durall DM, Tinker PB (1990) Phosphorus relationships and production of extramatrical hyphae of two types of willow ectomycorrhizas at different soil phosphorus levels. New Phytol 115:259–265
Jones MD, Durall DM, Tinker PB (1991) Fluxes of carbon and phosphorus between symbionts in willow estomycorrhizas and their changes with time. New Phytol 119:99–106
Jones MD, Tweig BD, Ward V, Barker J, Durall DM, Simard SW (2010) Functional complementarity of Douglas-fir ectomycorrhizas for extracellular enzyme activity after wildfire or clearcut logging. Funct Ecol 24:1139–1151
Kammenbauer H, Agerer R, Sanderman H (1989) Studies on ectomycorrhiza XXII. Mycorrhizal rhizomorphs of Telephora terrestris and Pisolithus tinctorius in association with Norway spruce (Picea abies): formation in vitro and translocation of phosphate. Trees 3:78–84
Kasurinen A, Keinanen MM, Kaipainen S, Nilsson LO, Vapaavuori E, Kontro MH, Holopainen T (2005) Below-ground responses of silver birch trees exposed to elevated CO2 and O-3 levels during three growing seasons. Glob Chang Biol 11:1167–1179
Khan FN, Lukac M, Turner G, Godbold DL (2008) Elevated atmospheric CO2 changes phosphorus fractions in soils under short rotation poplar plantation (EuroFACE). Soil Biol Biochem 40:1716–1723
Kieliszewska-Rokicka B (1992) Effect of nitrogen level on acid phosphatase activity of eight isolates of ectomycorrhizal fungus Paxillus involutus cultured in vitro. Plant Soil 139:229–238
Kieliszewska-Rokicka B (1999) Phosphate status and acid phosphatase activity in soil and ectomycorrhizas in two mature stands of Scots pine (Pinus sylvestris L.) exposed to different levels of anthropogenic pollution. Acta Soc Bot Pol 68:311–317
Klein DA, Paschke MW (2004) Filamentous fungi: the indeterminate lifestyle and microbial ecology. Microb Ecol 47:224–235
Kluber LA, Tinnesand KM, Caldwell BA, Dunham SM, Yarwood RR, Bottomley PJ, Myrold DD (2010) Ectomycorrhizal mats alter forest soil biogeochemistry. Soil Biol Biochem 42:1607–1613
Kogawara S, Norisada M, Tange T, Yagi H, Kojima K (2005) Elevated atmospheric CO2 concentration alters the effect of phosphate supply on growth of Japanese red pine (Pinus densiflora) seedlings. Tree Physiol 26:25–33
Koide RT (1991) Nutrient supply, nutrient demand and plant-response to mycorrhizal infection. New Phytol 117:365–386
Kothe E, Müller D, Krause K (2002) Different high affinity phosphate uptake systems of ectomycorrhizal Tricholoma species in relation to substrate specificity. J Appl Bot—Angew Bot 76:127–131
Kretzer AM, Dunham S, Molina R, Spatafora JW (2005) Patterns of vegetative growth and gene flow in Rhizopogon vinicolor and R. vesiculosis (Boletales, Basidiomycota). Mol Ecol 14:2259–2268
Kroehler CJ, Antibus RK, Linkins AE (1988) the effect of organic and inorganic phosphorus concentration on the acid phosphatase activity of ectomycorrhizal fungi. Can J Bot 66:750–756
Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ (2006) Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann Bot 98:693–713
Lambers H, Raven JA, Shaver GR, Smith SE (2008) Plant nutrient-acquisition strategies change with soil age. Trends Ecol Evol 23:95–103
Lambers H, Brundrett MC, Raven JA, Hopper SD (2010) Plant mineral nutrition in ancient landscapes: high plant species diversity on infertile soils is linked to functional diversity for nutritional strategies. Plant Soil 334:11–31
Landeweert R, Hoffland E, Finlay RD, Kuyper TW, van Breenan N (2001) Linking plants to rocks: ectomycorrhizal fungi mobilise nutrients from minerals. Trends Ecol Evol 16:248–254
Lapeyrie F, Chilvers GA, Bhem CA (1987) Oxalic acid synthesis by the ectomycorrhizal fungus Paxillus involutus. New Phytol 106:139–146
Lapeyrie F, Ranger J, Vairelles D (1991) Phosphate-solubilizing activity of ectomycorrhizal fungi in vitro. Can J Bot 69:342–346
Launonen TM, Ashton DH, Kelliher KJ, Keane PJ (2004) The growth and P acquisition of Eucalyptus regnans F. Muell. seedlings in air-dried and undried forest soil in relation to seedling age and ectomycorrhizal infection. Plant Soil 267:179–189
Lenburg ME, O’Shea EK (1996) Signalling phosphate starvation. Trends Biochem Sci 21:383–387
Leprince F, Quiquampoix H (1996) Extracellular enzyme activity in soil: effect of pH and ionic strength on the interaction with montmorillonite of two acid phosphatases secreted by the ectomycorrhizal fungus Hebeloma cylindrosporum. Eur J Soil Sci 47:511–522
Lewis JD, Thomas RB, Strain BR (1994) Effect of elevated CO2 on mycorrhizal colonization of loblolly pine (Pinus taeda L.) seedlings. Plant Soil 165:81–88
Lewis JD, Ward JK, Tissue DT (2010) Phosphorus supply drives nonlinear responses of cottonwood (Populus deltoides) to increase in CO2 concentration from glacial to future concentrations. New Phytol 187:438–448
Leyval C, Berthelin J (1986) Comparison between the utilization of phosphorus from insoluble mineral phosphates by ectomycorrhizal fungi. In: Gianinazzi-Pearson V, Gianinazzi S (eds) Physiological and genetical aspects of mycorrhizae. INRA, Nancy, pp 339–343
Lichko LP, Kulakovskaya TV, Kulaev IS (2006) Inorganic polyphosphates and exopolyphosphates in different cell compartments of Saccharomyces cerevisiae. Biochemistry (Moscow) 71:1171–1175
Lindahl B, Stenlid J, Olsson S, Finlay RD (1999) Translocation of 32P between interacting mycelia of a wood-decomposing fungus and ectomycorrhizal fungi in microcosm systems. New Phytol 144:183–193
Lindahl B, Stenlid J, Finlay RD (2001) Effects of resource availability on mycelial interactions and 32P transfer between a saprotrophic and an ectomycorrhizal fungus in soil microcosms. FEMS Microbiol Ecol 38:43–52
Lindahl BO, Taylor AFS, Finlay RD (2002) Defining nutritional constraints on carbon cycling in boreal forests—towards a less ‘phytocentric’ perspective. Plant Soil 242:123–135
Liu Q, Loganathan P, Hedley MJ (2005) Influence of ectomycorrhizal hyphae on phosphate fractions and dissolution of phosphate rock in rhizosphere soils of Pinus radiata. J Plant Nutr 28:1525–1540
Louche J, Ali MA, Cloutier-Hurteau B, Sauvage F-X, Quiquampoix H, Plassard C (2010) Efficiency of acid phosphatases secreted from the ectomycorrhizal fungus Hebeloma cylindrosporum to hydrolyse organic phosphorus in podzols. FEMS Microbiol Ecol 73:323–335
Malajczuk N, McComb AJ, Loneragan JF (1975) Phosphorus uptake and growth of mycorrhizal and uninfected seedlings of Eucalyptus calophylla R. Br. Aust J Bot 23:231–238
Marschner H (1995) Mineral nutrition of higher plants. Academic, London
Martin F, Nehls U (2009) Harnessing ectomycorrhizal genomics for ecological insights. Curr Opin Plant Biol 12:508–515
Martins A, Santos M, Santos H, Pais MS (1999) A 31P nuclear magnetic resonance study of phosphate levels in roots of ectomycorrhizal and nonmycorrhizal plants of Castanea sativa Mill. Trees 13:168–172
Marx DH, Hatch AB, Mandicino JF (1977) High soil fertility, sucrose content and susceptibility of loblolly pine roots to ectomycorrhizal infection by Pisolithus tinctorius. Can J Bot 55:1569–1574
Mason PA, Ingleby K, Munro RC, Wilson J, Ibrahim K (2000) The effect of reduced phosphorus concentration on mycorrhizal development and growth of Eucalyptus globulus Labill. seedlings inoculated with 10 different fungi. For Ecol Manag 128:249–258
McElhinney C, Mitchell DT (1993) Phosphatase activity of four ectomycorrhizal fungi found in a Sitka spruce-Japanese larch plantation in Ireland. Mycol Res 97:725–732
Melin E, Nilsson H (1950) Transfer of radioactive phosphorus to pine seedlings by means of mycorrhizal hyphae. Physiol Plant 3:88–92
Morris MH, Smith ME, Rizzo DM, Rejmánek M, Bledsoe CS (2008) Contrasting ectomycorrhizal fungal communities on the roots of co-occurring oaks (Quercus spp.) in a California woodland. New Phytol 178:167–176
Mosca E, Montecchio L, Scattolin L, Garbaye J (2007) Enzymatic activities of three ectomycorrhizal types of Quercus robur L. in relation to tree decline and thinning. Soil Biol Biochem 39:2897–2904
Mousain D, Salsac L (1986) Utilisation du phytate et activités phosphatases acides chez Pisolithus tinctorius, basidiomycete mycorhizien. Physiol Veg 24:193–200
Moyersoen B, Alexander IJ, Fitter AH (1998) Phosphorus nutrition of ectomycorrhizal and arbuscular mycorrhizal tree seedlings from a lowland tropical rain forest in Korup National Park, Cameroon. J Trop Ecol 14:47–61
Nguyen C, Yan W, Le Tacon F, Lapeyrie F (1992) Genetic variability of phosphate solubilizing activity by monocaryotic and dicaryotic mycelia of the ectomycorrhizal fungus Laccaria bicolor (Maire) P.D. Orton. Plant Soil 143:193–199
Nilsson L-O, Wallander H (2003) Production of external mycelium by ectomycorrhizal fungi in a Norway spruce forest was reduced in response to nitrogen fertilization. New Phytol 158:409–416
Norby RJ, O’Neill EG, Hood WG, Luxmore RJ (1987) Carbon allocation, root exudation and mycorrhizal colonization of Pinus echinata seedlings grown under CO2 enrichment. Tree Physiol 3:203–210
Norisada M, Motoshige T, Kojima K, Tange T (2006) Effects of phosphate supply and elevated CO2 on root acid phosphatase activity in Pinus densiflora seedlings. J Plant Nutr Soil Sci 169:274–279
Nygren CMR, Rosling A (2009) Localisation of phosphomonoesterase activity in ectomycorrhizal fungi grown on different phosphorus sources. Mycorrhiza 19:197–204
Orlovich DA, Ashford AE (1993) Polyphosphate granules are an artefact of specimen preparation in the ectomycorrhizal fungus Pisolithus tinctorius. Protoplasma 173:91–102
Pampolina NM, Dell B, Malajczuk N (2002) Dynamics of ectomycorrhizal fungi in an Eucalyptus globulus plantation: effect of phosphorus fertilization. For Ecol Manag 158:291–304
Parrent JL, Vilgalys R (2007) Biomass and compositional responses of ectomycorrhizal fungal hyphae to elevated CO2 and nitrogen fertilization. New Phytol 176:164–174
Parrent JL, Morris WF, Vilgalys R (2006) CO2-enrichment and nutrient availability alter ectomycorrhizal fungal communities. Ecology 87:2278–2287
Paul EA, Clark FE (1989) Soil microbiology and biochemistry. Academic, London
Perez-Moreno J, Read DJ (2000) Mobilization and transfer of nutrients from litter to tree seedlings via the vegetative mycelium of ectomycorrhizal plants. New Phytol 145:301–309
Perez-Moreno J, Read DJ (2001a) Exploitation of pollen by mycorrhizal mycelial systems with special reference to nutrient recycling in boreal forests. Proc R Soc Lond B 268:1329–1335
Perez-Moreno J, Read DJ (2001b) Nutrient transfer from soil nematodes to plants: a direct pathway provided by the mycorrhizal mycelial network. Plant Cell Environ 24:1219–1226
Peterson RL, Massicotte HB, Melville LH (2004) Mycorrhizas: anatomy and cell biology. CABI, Wallingford
Plassard C, Dell B (2010) Phosphorus nutrition of mycorrhizal trees. Tree Physiol 30:1129–1139
Pondugula S, Neef DW, Voth WP, Darst RP, Dhasarathy A, Reynolds MM, Takahata S, Stillman DJ, Kladde MP (2009) Coupling homeostasis to cell cycle-specific transcription: mitotic activation of Saccharomyces cerevisiae PHO5 by Mcm1 and forkhead proteins. Mol Cell Biol 29:4891–4905
Potila H, Wallander H, Sarjala T (2009) Growth of ectomycorrhizal fungi in drained peatland forests with variable P and K availability. Plant Soil 316:139–150
Pratt J, Boisson A-M, Gout E, Bligny R, Douce R, Aubert S (2009) Phosphate (Pi) starvation effects on the cytosolic Pi concentration and Pi exchanges across the tonoplast in plant cells: an in vivo 31P-nuclear magnetic resonance study using methylphosphonate as a Pi analog. Plant Physiol 151:1646–1657
Pritchard SG, Strand AE, McComack ML, Davis MA, Oren R (2008) Mycorrhizal and rhizomorph dynamics in a loblolly pine forest during 5 years of free-air-CO2-enrichment. Glob Chang Biol 14:1–13
Pritsch K, Raidl S, Marksteiner E, Blaschke H, Agerer R, Schloter M, Hartmann A (2004) A rapid and highly sensitive method for measuring enzyme activities in single mycorrhizal tips using 4-methylumbelliferone labelled fluorogenic substrates in a microplate system. J Microbiol Meth 58:233–241
Qu LY, Shinano T, Quoreshi AM, Tamai Y, Osaki M, Koike T (2004) Allocation of 14C-carbon in two species of larch seedlings infected with ectomycorrhizal fungi. Tree Physiol 24:1369–1376
Read DJ (1992) The mycorrhizal mycelium. In: Allen MF (ed) Mycorrhizal functioning. Chapman and Hall, New York, pp 102–132
Read DJ, Perez-Moreno J (2003) Mycorrhizas and nutrient cycling in ecosystems—a journay towards relevance. New Phytol 157:475–492
Redecker D, Szaro TM, Bowman RJ, Bruns TD (2001) Small genets of Lactarius xanthogalactus, Russula cremoricolor and Amanita francheti in late-stage ectomycorrhizal successions. Mol Ecol 10:1025–1034
Rees B, Shepherd VA, Ashford AE (1994) Presence of a motile tubular vacuole system in different phyla of fungi. Mycol Res 98:985–992
Reich PB, Hobbie SE, Lee T, Ellsworth DS, West JB, Tilman D, Knops JMH, Naeem S, Trost J (2006) Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nature 440:922–925
Rey A, Jarvis PG (1997) Growth responses of young birch trees (Betula pendula Roth.) after four and a half years of CO2 exposure. Ann Bot 80:809–816
Rineau F, Garbaye J (2009) Does forest liming impact the enzymatic profiles of ectomycorrhizal communities through specialized fungal symbionts? Mycorrhiza 19:493–500
Rolin D, Le Tacon F, Larher F (1984) Characterization of the different forms of phosphorus in the mycelium of the ectomycorrhizal fungus, Hebeloma cylindrosporum, cultivated in pure culture. New Phytol 98:335–343
Rosling A (2009) Trees, mycorrhiza and minerals—field relevance of in vitro experiments. Geomicrobiol J 26:389–401
Rosling A, Suttle KB, Johansson E, van Hees PAW, Banfield JF (2007) Phosphorus availability influences the dissolution of apatite by soil fungi. Geobiology 5:265–280
Rouhier H, Read DJ (1998) Plant and fungal responses to elevated atmospheric carbon dioxide in mycorrhizal seedlings of Pinus sylvestris. Environ Exp Bot 40:237–246
Rouhier H, Read DJ (1999) Plant and fungal responses to elevated atmospheric CO2 in mycorrhizal seedlings of Betula pendula. Environ Exp Bot 42:231–241
Rousseau JVD, Reid CPP (1991) Effects of phosphorus fertilization and mycorrhizal development on phosphorus nutrition and carbon balance of loblolly pine. New Phytol 117:319–326
Rousseau JVD, Sylvia DM, Fox AJ (1994) Contribution of ectomycorrhiza to the potential nutrient-absorbing surface of pine. New Phytol 128:639–644
Rygiewicz PT, Martin KJ, Tuininga AR (2000) Morphotype community structure of ectomycorrhizas on Douglas fir (Pseudotsuga menziesii Mirb. Franco) seedlings grown under elevated atmospheric CO2 and temperature. Oecologia 124:299–308
Sawyer NA, Chambers SM, Cairney JWG (1999) Molecular investigation of genet distribution and genetic variation of Cortinarius rotundisporus. New Phytol 142:561–568
Sawyer NA, Chambers SM, Cairney JWG (2003) Utilisation of inorganic and organic phosphorus sources by isolates of Amanita muscaria and Amanita species native to temperate eastern Australia. Aust J Bot 51:151–158
Shepherd VA, Orlovich DA, Ashford AE (1993a) A dynamic continuum of pleiomorphic tubules in growing hyphae of a fungus. J Cell Sci 104:495–507
Shepherd VA, Orlovich DA, Ashford AE (1993b) Cell-to-cell transport via motile tubules in growing hyphae of a fungus. J Cell Sci 105:1173–1178
Shinano T, Yamamoto T, Tawaraya K, Tadokoro M, Koike T, Osaki M (2007) Effects of elevated atmospheric CO2 concentration on the nutrient uptake characteristics of Japanese larch (Larix kaempferi). Tree Physiol 27:97–104
Simard SW, Jones MD, Durall DM (2002) Carbon and nutrient fluxes within and between mycorrhizal plants. In: van der Heijden MGA, Sanders IR (eds) Mycorrhizal ecology [ecological studies vol. 157]. Springer-Verlag, Berlin, pp 33–74
Skinner MF, Bowen GD (1974) The uptake and translocation of phosphate by mycelial strands of pine mycorrhizas. Soil Biol Biochem 6:53–56
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. Academic, Amsterdam
Smith SE, Gianinazzi-Pearson V, Koide R, Cairney JWG (1994) Nutrient transport in mycorrhizas: structure, physiology and consequences for the symbiosis. Plant Soil 159:103–113
olaiman MZ, Saito M (2001) Phosphate efflux from intraradical hyphae of Gigaspora margarita in vitro and its implication for phosphorus translocation. New Phytol 151:525–533
Sun Y-P, Unestam T, Lucas SD, Johanson KJ, Kenne L, Finlay RD (1999) Exudation-reabsorption in mycorrhizal fungi, the dynamic interface for interaction with soil and other microorganisms. Mycorrhiza 9:137–144
Taniguchi T, Kataoka R, Futai K (2008) Plant growth and nutrition in pine seedlings (Pinus thunbergia) seedlings and dehydrogenase and phosphatase activity of ectomycorrhizal root tips inoculated with seven individual ectomycorrhizal fungal species at high and low nitrogen conditions. Soil Biol Biochem 40:1235–1243
Tatry M-V, Kassis EE, Lambilliote R, Corratgé C, van Aarle I, Amenc LK, Alary R, Zimmermann S, Sentenac H, Plassard C (2009) Two differentially regulated transporters from the symbiotic fungus Hebeloma cylindrosporum and phosphorus acquisition by ectomycorrhizal Pinus pinaster. Plant J 57:1092–1102
Taylor JH, Peterson CA (1998) Viability and wall permeability of the extramatrical hyphae of the ectomycorrhizal fungus Hebeloma cylindrosporum. Can J Bot 76:893–898
Theodorou C (1971) The phytase activity of the mycorrhizal fungus Rhizopogon luteolus. Soil Biol Biochem 3:89–90
Thomas MR, O’Shea EK (2005) An intracellular phosphate buffer filters transient fluctuations in extracellular phosphate levels. Proc Natl Acad Sci USA 102:9565–9570
Thomson BD, Grove TS, Malajkzuk N, Hardy GES (1994) The effectiveness of ectomycorrhizal fungi in increasing the growth of Eucalyptus globulus Labill. in relation to root colonization and hyphal development. New Phytol 126:517–524
Tibbett M (2002) Considerations on the use of the p-nitrophenyl phosphomonoesterase assay in the study of the phosphorus nutrition of soil borne fungi. Microbiol Res 157:221–231
Tibbett M, Grantham K, Sanders FE, Cairney JWG (1998a) Induction of cold active phosphomonoesterase activity at low temperature in psychrotrophic ectomycorrhizal Hebeloma spp. Mycol Res 102:1533–1539
Tibbett M, Sanders FE, Cairney JWG (1998b) The effect of temperature and inorganic phosphorus concentration on acid phosphatase production and growth rate in arctic and temperate isolates of the ectomycorrhizal fungi Hebeloma spp. in axenic culture. Mycol Res 102:129–135
Tibbett M, Sanders FE, Grantham K, Cairney JWG (2000) Some potential inaccuracies of the p-nitrophenyl phosphomonoesterase assay in the study of the phosphorus nutrition of soil borne fungi. Biol Fertil Soils 31:92–96
Timonen S, Sen R (1998) Heterogeneity of fungal and plant enzyme expression in intact Scots pine-Suillus bovinus and -Paxillus involutus mycorrhizospheres developed in natural forest humus. New Phytol 138:355–366
Timonen S, Finlay RD, Olsson S, Söderström B (1996) Dynamics of phosphorus translocation in intact ectomycorrhizal systems: non-destructive monitoring using a β-scanner. FEMS Microbiol Ecol 19:171–180
Torres Aquino M, Plassard C (2004) Dynamics of ectomycorrhizal mycelial growth and P transfer to the host plant in response to low and high soil P availability. FEMS Microbiol Ecol 48:149–156
Tuason MMS, Arocena JM (2009) Calcium oxalate biomineralization by Piloderma fallax in response to various levels of calcium and phosphorus. Appl Environ Microbiol 75:7079–7085
Turner BJ (2008) Resource partitioning for soil phosphorus: a hypothesis. J Ecol 96:698–702
Unestam T, Sun Y-P (1995) Extramatrical structures of hydrophobic and hydrophilic ectomycorrhizal fungi. Mycorrhiza 5:301–311
Uroz S, Calvaruso C, Turpault MP, Pierrat JC, Mustin C, Frey-Klett P (2007) Effect of the mycorrhizosphere on the genotypic and metabolic diversity of the bacterial communities involved in mineral weathering in a forest soil. Appl Environ Microbiol 73:3019–3027
Uroz S, Calvaruso C, Turpault MP, Saringuet A, de Boer W, Leveau JHJ, Frey-Klett P (2009) Efficient mineral weathering is a distinctive functional trait of the bacterial genus Collimonas. Soil Biol Biochem 41:2178–2186
Van Aarle IM, Plassard C (2010) Spatial distribution of phosphatase activity associated with ectomycorrhizal plants is related to soil type. Soil Biol Biochem 42:324–330
Van Aarle IM, Viennois G, Amenc LK, Tatry M-V, Luu DT, Plassard C (2007) Fluorescent in situ RT-PCR to visualise the expression of a phosphate transporter gene from an ectomycorrhizal fungus. Mycorrhiza 17:487–494
van der Heijden EW, de Vries FW, Kuyper TW (1999) Mycorrhizal associations of Salix repens L. communities in succession of dune ecosystems. I. Above-ground and below-ground views of ectomycorrhizal fungi in relation to soil chemistry. Can J Bot 77:1821–1832
Van Hees PAW, Godbold DL, Jentschke G, Jones DL (2003) Impact of ectomycorrhizas on the concentration and biodegradation of simple organic acids in a forest soil. Eur J Soil Sci 54:697–706
Van Hees PAW, Rosling A, Essén S, Godbold DL, Jones DL, Finlay RD (2006) Oxalate and ferricrocin exudation by the extramatrical mycelium of an ectomycorrhizal fungus in symbiosis with Pinus sylvestris. New Phytol 169:367–378
Van Schöll L, Hoffland E, van Breenan N (2006) Organic acid exudation by ectomycorrhizal fungi and Pinus sylvestris in response to nutrient deficiencies. New Phytol 170:153–163
Van Schöll L, Kuyper TW, Smits MM, Landeweert R, Hoffland E, van Breenan N (2008) Rock-eating mycorrhizas: their role in plant nutrition and biogeochemical cycles. Plant Soil 303:35–47
Van Tichelen KK, Colpaert JV (2000) Kinetics of phosphate absorption by mycorrhizal and non-mycorrhizal Scots pine seedlings. Physiol Plant 110:96–103
Wallander H (2000) Uptake of P from apatite by Pinus sylvestris seedlings colonised by different ectomycorrhizal fungi. Plant Soil 218:249–256
Wallander H, Hagerberg D (2004) Do ectomycorhizal fungi have a significant role in weathering of minerals in forest soil? Symbiosis 27:249–257
Wallander H, Nylund J-E (1992) Effects of excess nitrogen and phosphorus starvation on the extramatrical mycelium of ectomycorrhizas of Pinus sylvestris L. New Phytol 120:495–503
Wallander H, Thelin G (2008) The stimulating effect of apatite on ectomycorrhizal growth diminishes after PK fertilization. Soil Biol Biochem 40:2517–2522
Wallander H, Wickman T, Jacks G (1997) Apatite as a P source in mycorrhizal and non-mycorrhizal Pinus sylvestris seedlings. Plant Soil 196:123–131
Wells JM, Boddy L (1995) Phosphorus translocation by saprotrophic basidiomycete cord systems on the floor of a mixed deciduous woodland. Mycol Res 99:977–980
Wykoff DD, Rizvi AH, Raser JM, Margolin B, O’Shea EK (2007) Positive feedback regulates switching of phosphate transporters in S. cerevisiae. Mol Cell 27:1005–1013
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Katharina Pawlowski.
Rights and permissions
About this article
Cite this article
Cairney, J.W.G. Ectomycorrhizal fungi: the symbiotic route to the root for phosphorus in forest soils. Plant Soil 344, 51–71 (2011). https://doi.org/10.1007/s11104-011-0731-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-0731-0