Abstract
Background and aims
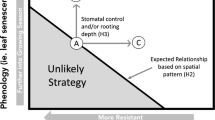
The survival and coexistence of plants in water-limited environments are related to their ability to coordinate water acquisition and regulation of water loss. To assess the coordination among below and aboveground hydraulic traits and the diversity of water-use strategies, we evaluated rooting depth and several leaf hydraulic traits of 15 species in campos rupestres, a seasonally-dry biodiversity hotspot in central Brazil.
Methods
We assessed the depth of plant water acquisition by excavating roots and analyzing the stable isotope composition of hydrogen (δD) and oxygen (δ18O) in the xylem and soil water. We also measured mid-morning stomatal conductance, leaf-water potential at turgor loss point (ѰTLP) and pre-dawn leaf water potentials (ѰPD) during wet and dry seasons.
Results
We demonstrated that rooting depth is a good predictor of seasonal variations in stomatal conductance and ѰPD. Shallow-rooted plants had greater variation in stomatal conductance and ѰPD than deep-rooted plants. Woody plants with shallower roots also had lower ѰTLP than deep-rooted plants, revealing higher drought resistance.
Conclusion
We demonstrate that shallow-rooted species, more exposed to variation in water availability, have mechanisms to confer drought resistance through turgor maintenance. Our results support the theory of hydrological niche segregation and its underlying trade-offs related to drought resistance.





Similar content being viewed by others
References
Abrahão A, Lambers H, Sawaya ACHF, Mazzafera P, Oliveira RS (2014) Convergence of a specialized root trait in plants from nutrient-impoverished soils: phosphorus-acquisition strategy in a nonmycorrhizal cactus. Oecologia 176:345–355. doi:10.1007/s00442-014-3033-4
Aguinis H, Gottfredson RK, Joo H (2013) Best-practice recommendations for defining, identifying and handling outliers. Organ Res Methods 16:270–301. doi:10.1177/1094428112470848
Alcantara S, Mello-Silva R, Teodoro GS, Drequeceler K, Ackerly DD, Oliveira RS (2015) Carbon assimilation and habitat segregation in resurrection plants: a comparison between desiccation-and non-desiccation-tolerant species of Neotropical Velloziaceae (Pandanales). Funct Ecol 29:1499–1512. doi:10.1111/1365-2435.12462
Apezzato-da-Glória B (2003) Morfologia de sistemas subterrâneos. ESALQ/USP, Ribeirao Preto
Araya YN, Silvertown J, Gowing DJ, McConway KJ, Linder HP, Midgley G (2011) A fundamental, eco-hydrological basis for niche segregation in plant communities. New Phytol 189:253–258. doi:10.1111/j.1469-8137.2010.03475.x
Bartelheimer M, Gowing D, Silvertown J (2010) Explaining hydrological niches: the decisive role of below-ground competition in two closely related Senecio species. J Ecol 98:126–136. doi:10.1111/j.1365-2745.2009.01598.x
Bartlett MK, Scoffoni C, Sack L (2012) The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: a global meta-analysis. Ecol Lett 15:393–405. doi:10.1111/j.1461-0248.2012.01751.x
Bates D, Machler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Blondel J (2003) Guilds or functional groups: does it matter? Oikos 100:223–231. doi:10.1034/j.1600-0706.2003.12152.x
Bond WJ, Midgley JJ (2003) The evolutionary ecology of sprouting in woody plants. Int J Plant Sci 164:103–114
Brodribb TJ, Holbrook NM, Edwards EJ, Gutierrez MV (2003) Relations between stomatal closure, leaf turgor and xylem vulnerability in eight tropical dry forest trees. Plant Cell Environ 26:443–450. doi:10.1046/j.1365-3040.2003.00975.x
Cade BS, Noon BR (2003) A gentle introduction to quantile regression for ecologists. Front Ecol Environ 1:412–420. doi:10.1890/1540-9295(2003)001[0412:AGITQR]2.0.CO;2
Castro SAB, Silveira FAO, Marcato MS, Lemos-Filho JP (2016) So close, yet so different: divergences in resource use may help stabilize coexistence of phylogenetically-related species in a megadiverse grassland. Flora-Morphology, Distribution, Functional Ecology of Plants doi:10.1016/j.flora.2016.11.018
Cosme LHM, Schietti J, Costa FRC, Oliveira RS (2017) The importance of hydraulic architecture to the distribution pattern of trees in a central Amazonian forest. New Phytol 215:113–125. doi:10.1111/nph.14508
Dawson TE, Ehleringer JR (1998) Plants, isotopes and water use: a catchment-scale perspective. In: Kendall C, McDonnell J (eds) Isotope tracers in catchment hydrology. Elsevier, Amsterdam
Dawson TE, Pate JS (1996) Seasonal water uptake and movement in root systems of Australian phraeatophytic plants of dimorphic root morphology: a stable isotope investigation. Oecologia 107:13–20. doi:10.1007/bf00582230
Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP (2002) Stable isotopes in plant ecology. Annu Rev Ecol Syst 33:507–559
de Souza A, de Moraes MG, Ribeiro RCLF (2005) Gramíneas do cerrado: carboidratos não-estruturais e aspectos ecofisiológicos. Acta Bot Bras 19:81–90
Díaz S, Kattge J, Cornelissen JHC, Wright IJ, Lavorel S, Dray S, Reu B, Kleyer M, Wirth C, Prentice IC (2016) The global spectrum of plant form and function. Nature 529:167–171. doi:10.1038/nature16489
Dietrich RC, Bengough AG, Jones HG, White PJ (2013) Can root electrical capacitance be used to predict root mass in soil? Ann Bot 112:457–464. doi:10.1093/aob/mct044
Ding Y, Zhang Y, Zheng Q-S, Tyree MT (2014) Pressure-volume curves: revisiting the impact of negative turgor during cell collapse by literature review and simulations of cell micromechanics. New Phytol 203:378–387. doi:10.1111/nph.12829
Donovan LA, Linton MJ, Richards JH (2001) Predawn plant water potential does not necessarily equilibrate with soil water potential under well-watered conditions. Oecologia 129:328–335. doi:10.1007/s004420100738
Donovan LA, Richards JH, Linton MJ (2003) Magnitude and mechanisms of disequilibrium between predawn plant and soil water potentials. Ecology 84:463–470. doi:10.1890/0012-9658(2003)084[0463:MAMODB]2.0.CO;2
Ferri MG (1944) Transpiração de plantas permanentes dos “cerrados”. Bol Fac Filos Ciênc Univ São Paulo 41:161–224
Garcia RJF, Longhi-Wagner HM, Pirani JR, Meirelles ST (2009) A contribution to the phytogeography of Brazilian campos: an analysis based on Poaceae. Brazilian J Bot 32:703–713. doi:10.1590/s0100-84042009000400009
Giulietti AM, De Menezes NL, Pirani JR, Meguro M, Wanderley MDGL (1987) Flora da Serra do Cipó, Minas Gerais: caracterização e lista das espécies. Boletim de Botânica da Universidade de São Paulo 9:1–151
Gonfiantini R (1978) Standards for stable isotope measurements in natural compounds. Nature 271:534–536. doi:10.1038/271534a0
Ivanov VY, Hutyra LR, Wofsy SC, Munger JW, Saleska SR, de Oliveira RC Jr, de Camargo PB (2012) Root niche separation can explain avoidance of seasonal drought stress and vulnerability of overstory trees to extended drought in a mature Amazonian forest. Water Resour Res 48. doi:10.1029/2012wr011972
Jackson RB, Canadell J, Ehleringer JR, Mooney HA, Sala OE, Schulze ED (1996) A global analysis of root distributions for terrestrial biomes. Oecologia 108:389–411. doi:10.1007/bf00333714
Jacobi CM, do Carmo FF, Vincent RC, Stehmann JR (2007) Plant communities on ironstone outcrops: a diverse and endangered Brazilian ecosystem. Biodivers Conserv 16:2185–2200. doi:10.1007/s10531-007-9156-8
Klein T (2014) The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Funct Ecol 28:1313–1320. doi:10.1111/1365-2435.12289
Koenker R (2017) Quantreg: quantile regression. R package version 5.33. R Foundation for Statistical Computing, Vienna. Available at: http://CRAN.R-project.org/package=quantreg
Küppers M, Neales TF, Küppers BIL, Swan AG, Myers BA (1987) Hydraulic flow characteristics in the lignotuberous mallee Eucalyptus behriana F. Muell in the field. Plant Cell Environ 10:27–37. doi:10.1111/j.1365-3040.1987.tb02076.x
Le Stradic S, Buisson E, Fernandes GW (2015) Vegetation composition and structure of some Neotropical mountain grasslands in Brazil. J Mt Sci 12:864–877. doi:10.1007/s11629-013-2866-3
Lenth RV (2016) Least-squares means: the R package lsmeans. J Stat Softw 69:1–33. doi:10.18637/jss.v069.i01
Lenz TI, Wright IJ, Westoby M (2006) Interrelations among pressure-volume curve traits across species and water availability gradients. Physiol Plant 127:423–433. doi:10.1111/j.1399-3054.2006.00680.x
Letten AD, Keith DA, Tozer MG, Hui FKC (2015) Fine-scale hydrological niche differentiation through the lens of multi-species co-occurrence models. J Ecol 103:1264–1275. doi:10.1111/1365-2745.12428
Meinzer FC, Woodruff DR, Eissenstat DM, Lin HS, Adams TS, McCulloh KA (2013) Above- and belowground controls on water use by trees of different wood types in an eastern US deciduous forest. Tree Physiol 33:345–356. doi:10.1093/treephys/tpt012
Meinzer FC, Woodruff DR, Marias DE, Smith DD, McCulloh KA, Howard AR, Magedman AL (2016) Mapping ‘hydroscapes’ along the iso-to anisohydric continuum of stomatal regulation of plant water status. Ecol Lett 19:1343–1352. doi:10.1111/ele.12670
Mitchell PJ, O'Grady AP, Pinkard EA, Brodribb TJ, Arndt SK, Blackman CJ, Duursma RA, Fensham RJ, Hilbert DW, Nitschke CR (2016) An ecoclimatic framework for evaluating the resilience of vegetation to water deficit. Glob Chang Biol. doi:10.1111/gcb.13177
Myers BA (1995) The influence of the lignotuber on hydraulic conductance and leaf conductance in Eucalyptus behriana seedlings. Aust J Plant Physiol 22:857–863
Nepstad DC, de Carvalho CR, Davidson EA, Jipp PH, Lefebvre PA, Negreiros GH, da Silva ED, Stone TA, Trumbore SE, Vieira S (1994) The role of deep roots in the hydrological and carbon cycles of Amazonian forests and pastures. Nature 372:666–669. doi:10.1038/372666a0
Nie Y-p, Chen H-s, Wang K-l, Tan W, Deng P-y, Yang J (2011) Seasonal water use patterns of woody species growing on the continuous dolostone outcrops and nearby thin soils in subtropical China. Plant Soil 341:399–412. doi:10.1007/s11104-010-0653-2
Oliveira RS, Bezerra L, Davidson EA, Pinto F, Klink CA, Nepstad DC, Moreira A (2005a) Deep root function in soil water dynamics in cerrado savannas of central Brazil. Funct Ecol 19:574–581. doi:10.1111/j.1365-2435.2005.01003.x
Oliveira RS, Dawson TE, Burgess SSO, Nepstad DC (2005b) Hydraulic redistribution in three Amazonian trees. Oecologia 145:354–363. doi:10.1007/s00442-005-0108-2
Oliveira RS, Christoffersen BO, Barros FV, Teodoro GS, Bittencourt P, Brum MMJ, Viani RAG (2014) Changing precipitation regimes and the water and carbon economies of trees. Theor Exp Plant Physiol 26:65–82. doi:10.1007/s40626-014-0007-1
Oliveira RS, Galvao HC, de Campos MCR, Eller CB, Pearse SJ, Lambers H (2015) Mineral nutrition of campos rupestres plant species on contrasting nutrient-impoverished soil types. New Phytol 205:1183–1194. doi:10.1111/nph.13175
Oliveira RS, Abrahão A, Pereira C, Teodoro GS, Brum M, Alcantara S, Lambers H (2016) Ecophysiology of Campos Rupestres plants. In: Fernandes GW (ed) Ecology and conservation of mountaintop grasslands in Brazil. Springer International Publishing
Parnell A, Jackson A (2013) Siar: stable isotope analysis in R. R package version 4.2. Available from: http://CRAN.R-project.org/package=siar. Accessed 23 March 2014
Parnell AC, Phillips DL, Bearhop S, Semmens BX, Ward EJ, Moore JW, Jackson AL, Grey J, Kelly DJ, Inger R (2013) Bayesian stable isotope mixing models. Environmetrics 24:387–399. doi:10.1002/env.2221
Pivovaroff AL, Pasquini SC, De Guzman ME, Alstad KP, Stemke JS, Santiago LS (2015) Multiple strategies for drought survival among woody plant species. Funct Ecol. doi:10.1111/1365-2435.12518
Poot P, Lambers H (2003) Are trade-offs in allocation pattern and root morphology related to species abundance? A congeneric comparison between rare and common species in the south-western Australian flora. J Ecol 91:58–67. doi:10.1046/j.1365-2745.2003.00738.x
Porembski S, Barthlott W (2000) Granitic and gneissic outcrops (inselbergs) as centers of diversity for desiccation-tolerant vascular plants. Plant Ecol 151:19–28. doi:10.1023/a:1026565817218
Quesada CA, Hodnett MG, Breyer LM, Santos AJB, Andrade S, Miranda HS, Miranda AC, Lloyd J (2008) Seasonal variations in soil water in two woodland savannas of central Brazil with different fire history. Tree Physiol 28:405–415. doi:10.1093/treephys/28.3.405
Romero R, Nakajima JN (1999) Espécies endêmicas do Parque Nacional da Serra da Canastra, Minas Gerais. Rev Bras Bot 22:259–265
Rossatto DR, Sternberg LSL, Franco AC (2013) The partitioning of water uptake between growth forms in a Neotropical savanna: do herbs exploit a third water source niche? Plant Biol 15:84–92. doi:10.1111/j.1438-8677.2012.00618.x
Sarmiento G, Monasterio M (1983) Life forms and phenology. In: Bourliere F (ed) Ecosystems of the world. Elsevier, Amsterdam
Schenk HJ, Jackson RB (2002) Rooting depths, lateral root spreads and below-ground/above-ground allometries of plants in water-limited ecosystems. J Ecol 90:480–494. doi:10.1046/j.1365-2745.2002.00682.x
Schwinning S (2010) The ecohydrology of roots in rocks. Ecohydrology 3:238–245. doi:10.1002/eco.134
Schwinning S, Ehleringer JR (2001) Water use trade-offs and optimal adaptations to pulse-driven arid ecosystems. J Ecol 89:464–480. doi:10.1046/j.1365-2745.2001.00576.x
Schwinning S, Kelly CK (2013) Plant competition, temporal niches and implications for productivity and adaptability to climate change in water-limited environments. Funct Ecol 27:886–897. doi:10.1111/1365-2435.12115
Schwinning S, Sala OE, Loik ME, Ehleringer JR (2004) Thresholds, memory, and seasonality: understanding pulse dynamics in arid/semi-arid ecosystems. Oecologia 141:191–193. doi:10.1007/s00442-004-1683-3
Silveira FAO, Negreiros D, Barbosa NPU, Buisson E, Carmo FF, Carstensen DW, Conceição AA, Cornelissen TG, Echternacht L, Fernandes GW, Garcia QS, Guerra TJ, Jacobi CM, Lemos-Filho JP, Le Stradic S, Morellato LPC, Neves FS, Oliveira RS, Schaefer CE, Viana PL, Lambers H (2016) Ecology and evolution of plant diversity in the endangered campo rupestre: a neglected conservation priority. Plant Soil 1–24. doi:10.1007/s11104-015-2637-8
Silvertown J, Dodd ME, Gowing DJG, Mountford JO (1999) Hydrologically defined niches reveal a basis for species richness in plant communities. Nature 400:61–63. doi:10.1038/21877
Silvertown J, Araya Y, Gowing D (2015) Hydrological niches in terrestrial plant communities: a review. J Ecol 103:93–108. doi:10.1111/1365-2745.12332
Team RC (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Turner NC (1988) Measurement of plant water status by the pressure chamber technique. Irrig Sci 9:289–308. doi:10.1007/bf00296704
West AG, Dawson TE, February EC, Midgley GF, Bond WJ, Aston TL (2012) Diverse functional responses to drought in a Mediterranean-type shrubland in South Africa. New Phytol 195:396–407. doi:10.1111/j.1469-8137.2012.04170.x
Acknowledgements
We gratefully acknowledge CAPES agency for the scholarships granted to MB and AA; financial support by the São Paulo Research Foundation (FAPESP) with a scholarship to GST (2010/50327-8 and 2012/21015-3) and grant to RSO (2010/10204-0; 2011/52072-0). We thank Dr. Lucy Rowland for reviewing the manuscript; Caroline S. Muller and José Carmelo for helping in the field work, Dr. Plinio B. de Camargo, Dr. Marcelo Z. Moreira and Mr. Geraldo de Arruda for allowing the use of laboratory facilities at CENA-USP, ICMBio for allowing this study at PNSC, and Canastra Adventure and Dona Vicentina for the logistic support. The authors confirm do not have conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Richard Whalley.
Electronic supplementary material
ESM 1
(DOCX 2509 kb)
Rights and permissions
About this article
Cite this article
Brum, M., Teodoro, G.S., Abrahão, A. et al. Coordination of rooting depth and leaf hydraulic traits defines drought-related strategies in the campos rupestres, a tropical montane biodiversity hotspot. Plant Soil 420, 467–480 (2017). https://doi.org/10.1007/s11104-017-3330-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3330-x