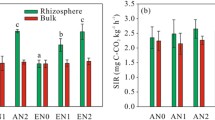
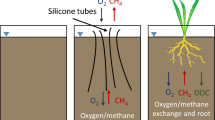
Abstract
Aims
The major factors controlling the soil methane (CH4) concentration and CH4 emissions of various plant (mainly wetland) species were identified.
Methods
Five plant species (Oryza sativa, Zizania latifolia, Phragmites australis, Sesbania cannabina, and Bidens tripartita) were separately cultivated under the flooded soil conditions. The direct CH4 scavenging potential of B. tripartita roots was also measured in conjunction with in vitro CH4 scavenging experiments using H2O2 and several transition metal ions.
Results
CH4 emissions from the soil-cultivated plants did not depend on the maximum CH4 emission potential for each plant species but on the soil CH4 concentrations, which were positively correlated with the CH4 production potential of the soil and negatively correlated with soil Eh values. Bidens tripartita roots possessed the highest increasing soil Eh potential and a direct, immediate, and continuous CH4 scavenging potential via the Fenton reaction using a considerably high concentration of root apoplastic H2O2 and rhizosphere Fe2+.
Conclusions
Bidens tripartita presented the highest soil Eh ascending potential. The in vitro experiments suggested the involvement of・OH/FeIVO2+ via the newly termed rhizosphere Fenton reaction as a strong destructive power for CH4. To our knowledge, this is the first report on direct CH4 scavenging by high H2O2-exuding plant roots.










Similar content being viewed by others
Abbreviations
- α-NA:
-
α-naphthylamine
- Eh:
-
Oxidation-reduction potential
- FeIVO2+ :
-
Ferryl ion
- OH:
-
Hydroxyl radical
References
Akhter A, Khan MSH, Egashira H, Tawaraya K, Rao IM, Wenzl P, Ishikawa S, Wagatsuma T (2009) Greater contribution of low-nutrient tolerance to sorghum and maize growth under combined stress conditions with high aluminum and low nutrients in solution culture simulating the nutrient status of tropical acid soils. Soil Sci Plant Nutr 55:394–406. https://doi.org/10.1111/j.1747-0765.2009.00372.x
Ando T, Yoshida S, Nishiyama I (1983) Nature of oxidizing power of rice roots. Plant Soil 72:57–71
Armstrong J, Armstrong W (1988) Phragmites australis: a preliminary study of soil-oxidising sites and internal gas transport pathways. New Phytol 108:373–382
Armstrong J, Armstrong W (1990) Light-enhanced convective throughflow increases oxygenation in rhizomes and rhizosphere of Phragmites australis (Cav.) Trin. ex Steud. New Phytol 114:121–128
Bertoli AC, Garcia JS, Trevisan MG, Ramalho TC, Freitas MP (2016) Interactions fulvate-metal (Zn2+, Cu2+ and Fe2+): theoretical investigation of thermodynamic, structural and spectroscopic properties. Biometals 29:275–285
Bhullar GS, Edwards PJ, Venterink HO (2013a) Variation in the plant-mediated methane transport and its importance for methane emission from intact wetland peat mesocosms. J Plant Ecol 6:298–304
Bhullar GS, Iravani M, Edwards PJ, Venterink HO (2013b) Methane transport and emissions from soil affected by water table and vascular plants. BMC Ecol 13:32
Bhullar GS, Edwards PJ, Venterink HO (2014) Influence of different plant species on methane emissions from soil in a restored Swiss wetland. PLoS One 9:e89588
Bokare AD, Choi W (2014) Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J Hazard Mater 275:121–135
Bossmann SH, Oliveros E, Göb S, Siegwart S, Dahlen EP, Payawan L Jr, Straub M, Wörner M, Braun AM (1998) New evidence against hydroxyl radicals as reactive intermediates in the thermal and photochemically enhanced Fenton reactions. J Phys Chem A 102:5542–5550
Bridgham S, Cadillo-Quiroz H, Keller JK, Zhuang Q (2013) Methane emissions from wetlands: biogeochemical, microbial, and modeling perspective from local to global scales. Glob Chang Biol 19:1325–1346. https://doi.org/10.1111/gcb.12131
Budiman H, Nuryatini ZO (2015) Comparison between GC-TCD and GC-FID for the determination of propane in gas mixture. Procedia Chem 16:465–472
Buxton GV, Greenstock CL, Helman WP, Ross AB (1988) Critical review of rate constants for reactions of hydrated electrons, hydrogen atom and hydroxyl radicals (・OH/・O−) in aqueous solutions. J Phys Chem Ref Data 17:513–886
Chan ASK, Parkin TB (2001) Methane oxidation and production activity in soils from natural and agricultural ecosystems. J Environ Qual 30:1896–1903
Chen SX, Schopfer P (1999) Hydroxyl-radical production in physiological reactions. A novel function of peroxidase. Eur J Biochem 260:726–735. https://doi.org/10.1046/j.1432-1327.1999.00199.x
Chen CC, Dixon JB, Turner FT (1980) Iron coatings on rice roots: mineralogy and quantity influencing factors. Soil Sci Soc Am J 44:635–639
Chumakov A, Batalova V, Slizhov Y (2016) Electro-Fenton like reactions of transition metal ios with electrogenerated hydrogen peroxide. AIP Conf Proc 1772, 040004-1-040004-6 https://doi.org/10.1063/1.4964563
Cordoba-Pedregosa MC, Gonzalez-Reyes JA, Canadillas MS, Navas P, Cordoba F (1996) Role of apoplastic and cell-wall peroxidases on the stimulation of root elongation by ascorbate. Plant Physiol 112:1119–1125. https://doi.org/10.1104/pp.112.3.1119
Deguillaume L, Leriche M, Chaumerliac N (2005) Impact of radical versus non-radical pathway in the Fenton chemistry on the iron redox cycle in clouds. Chemosphere 60:718–724
Dlugokencky Ed (2019) Global CH4 monthly means at July 2019: 1858.6 ppb NOAA/ESRL (www.esrl.noaa.gov/gmd/ccgg/trends_ch4/)
Dutta K, Mukhopadhyay S, Bhattacharjee S, Chaudhuri B (2001) Chemical oxidation of methylene blue using a Fenton-like reaction. J Hazard Mater B84:57–71
Enami S, Sakamoto Y, Colussi AJ (2014) Fenton chemistry at aqueous interfaces. Proc Natl Acad Sci U S A 111:623–628
Ensing B, Buda F, Blöchl PE, Baerends EJ (2002) A car-Parrinello study of the formation of oxidizing intermediates from Fenton’s reagent in aqueous solution. Physiol Chem Phys 4:3619–3627
Ensing B, Buda F, Gribnau MCM, Baerends EJ (2004) Methane-to-methanol oxidation by the hydrated iron (IV) oxo species in aqueous solution: a combined DFT and car-Parrinello molecular dynamics study. J Am Chem Soc 126:4355–4365
Esaki I, Watanabe A, Kimura M (1993) Water-soluble organic materials in paddy soil ecosystem. I. Fractionation of water-soluble organic materials in leachate from submerged paddy soils using PVP and ion exchange resins. Soil Sci Plant Nutr 39:529–538
Fletcher SEM, Schaefer H (2019) Rising methane: a new climate challenge. The amount of the greenhouse gas methane in Earth’s atmosphere is rising rapidly. Science 364:932–933
Frahry G, Schopfer P (1998) Hydrogen peroxide production by roots and its stimulation by exogenous NADH. Physiol Plant 103:395–404
Fu Y-G, Yang X-J, Ye Z-H, Shen H (2016) Identification, separation and component analysis of reddish brown and non-reddish brown iron plaque on rice (Oryza sativa) root surface. Plant Soil 402:277–290
Halliwell B (1989) Current status review. Free radicals, reactive oxygen species and human disease: a critical evaluation with special reference to atherosclerosis. Br J Exp Pathol 70:737–757
Hirata A, Sukizaki S, Suzuki S, Arata N, Nakamura S (2013) Acute toxicity of methane on marine organisms. J Adv Mar Sci Technol Soc 19:5–13
Hooijmaijers C, Rhee JY, Kwak KJ, Chung GC, Horie T, Katsuhara M, Kang H (2012) Hydrogen peroxide permeability of plasma membrane aquaporins of Arabidopsis thaliana. J Plant Res 125:147–153
Jiang Y, van GroenigenKJ HS, Hungate BA, van Kessel C, Hu S, Zhang J, Wu L, Yan X, Wang L, Chen J, Hang X, Zhang Y, Horwath WR, Ye R, Linquist BA, Song Z, Zheng C, Deng A, Zhang W (2017) Higher yields and lower methane emissions with new rice cultivars. Glob Chang Biol 23:4728–4738
Jiang Y, Quian H, Huang S, Zhang X, Wang L, Zhang L, Shen M, Xiao X, Chen F, Zhang H, Lu C, Zhang J, Deng A, van Groenigen KJ, Zhang W (2019) Acclimation of methane emissions from rice paddy fields to straw addition. Sci Adv 5:eaau9038
Kao-Kniffin J, Freyre DS, Balser TC (2010) Methane dynamics across wetland plant species. Aquat Bot 93:107–113
Kightley D, Nedwell DB , Cooper M (1995) Capacity for methane oxidation in landfill cover soils measured in laboratory-scale soil microcosms. Appl Environ Microbiol 61 (2):592–601
Kimura M, Minoda T, Murase J (1993) Water-soluble organic materials in paddy ecosystem. II. Effects of temperature on contents of total organic materials, organic acids, and methane in leachate from submerged paddy soils amended with rice straw. Soil Sci Plant Nutr 39:713–724
King GM (1994) Associations of methanotrophs with the roots and rhizomes of aquatic vegetation. Appl Environ Microbiol 60:3220–3227
Kirschke S, Bousquet P, Ciais P, Saunois M, Canadell JG, Dlugokenchy EJ, Bergamaschi P, Bergmann D, Blake DR, Bruhwiler L, Cameron-Smith P, Castaldi S, Chevallier F et al (2013) Three decades of global methane sources and sinks. Nat Geosci 6:813–823
Kiuchi T (1980) Measurement of α-naphthylamine oxidation potential of the plant roots. In: Committee of Crop Plant Analysis (ed) Analyzing methods for the cultivated plants. Yokendo, Tokyo, pp 531–533 (in Japanese)
Koelbener A, Ström L, Edwards PJ, Venterink HO (2010) Plant species from mesotrophic wetlands cause relatively high methane emissions from peat soil. Plant Soil 326:147–157
Koppenol WH, Liebman JF (1984) The oxidizing nature of the hydroxyl radical. A comparison with the ferryl ion (FeO2+). J Phys Chem 88:99–101
Kumagai K, Yagi K, Tsuta H, Minami K (1993) The measurement of methane production and oxidation in paddy soils. Jap J Soil Sci Plant Nutr 64:431–434 (in Japanese)
Lindau CW, Bollich PK, Delaune RD, Patrick WH Jr, Law VJ (1991) Effect of urea fertilizer and environmental factors on CH4 emissions from a Louisiana, USA rice field. Plant Soil 136:195–203
Lu Y, Wassmann R, Neue H-U, Huang C (2000) Dynamics of dissolved organic carbon and methane emissions in a flooded rice soil. Soil Sci Soc Am J 64:2011–2017
Maasakkers JD, Jacob DJ, Sulprizio MP, Scarpelli TR, Nesser H, Sheng J-X, Zhang Y, Hersher M, Bloom AA, Bowman KW, Worden JR, Janssens-Maenhout G, Parker RJ (2019) Global distribution of methane emissions, emission trends, and ・OH concentrations and trends inferred from an inversion of GOSAT satellite data for 2010-2015. Atmos Chem Phys 19:7859–7881
Matsubara C, Takamura K (1980) A new spectrophotometric method for the determination of trace of hydrogen peroxide by the titanium (IV)-4-(2-pyridylazo)-resorcinol reagent: application to the assay for hydrogen peroxide as a food additive. Bunseki Kagaku 29:759–764 (in Japanese with English summary)
Matsunaka S (1960) Stidies on the respiratory enzyme systems of plants I. Enzymatic oxidation of α-naphthylamine in rice plant root. J Biochem 47:820–829
McKay G, Kleinman JL, Johnston KM, Dong MM, Rosario-Ortiz FL, Mezyk SP (2014) Kinetics of the reaction between the hydroxyl radical and organic matter standards from the international humic substance society. J Soils Sediments 14:298–304
Møller IM, Jensen PE, Hansson A (2007) Oxidative modifications to cellular components in plants. Annu Rev Plant Biol 58:459–481
Nouchi I, Mariko S, Aoki K (1990) Mechanism of methane transport from the rhizosphere to the atmosphere through rice plants. Plant Physiol 94:59–66
Sagi M, Fluhr R (2006) Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol 141:336–340
Satoh AY, Trosko JE, Masten SJ (2007) Methylene blue dye test for rapid qualitative detection of hydroxyl radicals formed in a Fenton’s reaction aqueous solution. Environ Sci Technol 41:2881–2887
Saunois M, Bousquet P, Poulter B, Peregon A, Ciais P, Canadell JG, Dlugokencky EJ, Etiope G, Bastviken D, Houweling S, Janssens-Maenhout G, Tubiello FN, Castaldi S, Jackson RB, Alexe M, Arora VK, Beerling DJ, Bergamaschi P, Blake DR, Brailsford G, Brovkin V, Bruhwiler L, Crevoisier C, Crill P, Covey K, Curry C, Frankenberg C, Gedney N, Höglund-Isaksson L, Ishizawa M, Ito A, Joos F, Kim HS, Kleinen T, Krummel P, Lamarque JF, Langenfelds R, Locatelli R, Machida T, Maksyutov S, McDonald KC, Marshall J, Melton JR, Morino I, Naik V, O'Doherty S, Parmentier FJW, Patra PK, Peng C, Peng S, Peters GP, Pison I, Prigent C, Prinn R, Ramonet M, Riley WJ, Saito M, Santini M, Schroeder R, Simpson IJ, Spahni R, Steele P, Takizawa A, Thornton BF, Tian H, Tohjima Y, Viovy N, Voulgarakis A, van Weele M, van der Werf GR, Weiss R, Wiedinmyer C, Wilton DJ, Wiltshire A, Worthy D, Wunch D, Xu X, Yoshida Y, Zhang B, Zhang Z, Zhu Q (2016) The global methane budget 2000-2012. Earth Syst Sci Data 8:697–751
Schopfer P (1994) Histochemical demonstration and localization of H2O2 in organs of higher plants by tissue printing on nitrocellulose paper. Plant Physiol 104:1269–1275
Shannon RD, White JR, Lawson JE, Gilmour BS (1996) Methane efflux from emergent vegetation in peatlands. J Ecol 84:239–246
Shen B, Jensen RG, Bohnert HJ (1997) Increased resistance to oxidative stress in transgenic plants by targeting mannitol biosynthesis to chloroplasts. Plant Physiol 113:1177–1183
Shiba H, Daimon H (2003) Histological observation of secondary aerenchyma formed immediately after flooding in Sesbania cannabina and S. rostrata. Plant Soil 255:209–215
Sitaula BK, Hansen S, Sitaula JIB, Bakken LR (2000) Methane oxidation potentials and fluxes in agricultural soil: effects of fertilization and soil compaction. Biogeochemistry 48:323–339
Smirnoff N, Arnaud D (2019) Hydrogen peroxide metabolism and functions in plants. New Phytol 221:1197–1214
Srivastava V, Schinkel H, Witzell J, Hertzberg M, Torp M, Srivastava MK, Karpinska B, Melzer M, Wingsle G (2006) Downregulation of high-isoelectric-point extracellular superoxide dismutase mediates alterations in the metabolism of reactive oxygen species and developmental disturbances in hybrid aspen. Plant J 49:135–148
Sun Y, Pignatello JJ (1992) Chemical treatment of pesticide wastes. Evaluation of Fe(III) chelates for catalytic hydrogen peroxide oxidation of 2,4-D at circumneutral pH. J Agric Food Chem 40:322–327
Sundh I, Borga P, Nilsson M, Svensson BH (1995) Estimation of cell numbers of methanotrophic bacteria in boreal peatlands based on analysis of specific phospholipid fatty acids. FEMS Microbiol Ecol 18:103–112
van der Nat FJWA, Middelburg JJ, van Meteren D, Wielemakers A (1998) Diel methane emission patterns from Scirpus lacustris and Phragmites australis. Biogeochemistry 41:1–22
Vione D, Merlo F, Maurino V, Minero C (2004) Effect of humic acids on the Fenton degradation of phenol. Environ Chem Lett 2:129–133. https://doi.org/10.1007/s10311-004-0086-3
Wagatsuma T, Nakashima T, Tawaraya K, Watanabe S, Kamio A, Ueki A (1990) Relationship between wet tolerance, anatomical structure of aerenchyma and gas exchange ability among several plant species. Bull Yamagata Univ Agr Sci 11:121–141
Wagatsuma T, Jujo K, Tawaraya K, Sato T, Ueki A (1992) Decrease of methane concentration and increase of nitrogen gas concentration in the rhizosphere by hygrophytes. Soil Sci Plant Nutr 38:467–476
Wagatsuma T, Khan MSH, Watanabe T, Maejima E, Sekimoto H, Yokota T, Nakano T, Toyomasu T, Tawaraya K, Koyama H, Uemura M, Ishikawa S, Ikka T, Ishikawa A, Kawamura T, Murakami S, Ueki N, Umetsu A, Kannari T (2015) Higher sterol content regulated by CYP51 with concomitant lower phospholipid content in membranes is a common strategy for aluminum tolerance in several plant species. J Exp Bot 66:907–918
Wang B, Neue HU, Samonte HP (1997) Role of rice in mediating methane emission. Plant Soil 189:107–115
Wieczorek AS, Drake HL, Kolb S (2011) Organic acids and ethanol inhibit the oxidation of methane by mire methanotrophs. FEMS Microbiol Ecol 77:28–39
Xu J, Yang S, Peng S, Wei Q, Gao X (2013) Solubility and leaching risks of organic carbon in paddy soils as affected by irrigation managemrnts. The Scientific World Journal 2013:9 Article ID 546750 https://doi.org/10.1155/2013/546750
Yajima H (2015) Mechanism of the color development of iodine-starch reaction. Chem Educ 63:228–231 (in Japanese)
Yang J, Tam NF-Y, Ye Z (2014) Root porosity, radial oxygen loss and iron plaque on roots of wetland plants in relation to zinc tolerance and accumulation. Plant Soil 374:815–828
Yoshizawa K, Yamabe T (1997) Searching for the reaction mechanism on the conversion from methane to methanol – aim to model enzyme. Chemistry 52:36–41 (in Japanese)
Zheng H, Fu Z, Zhong J, Long W (2018) Low methane emission in rice cultivars with high radial oxygen loss. Plant Soil 431:1–10
Zhu L, Zhao R, Wang K, Xiang H, Shang Z, Sun W (2008) Electrochemical behaviors of methylene blue on DNA modified electrode and its application to the detection of PCR product from NOS sequence. Sensors 8:5649–5660
Acknowledgments
This work was partly supported by JSPS KAKENHI Grant-in-Aid for Scientific Research (18658026). We thank Bs.Sc. Taichi Itou, Hiroshi Takahashi, Syuuko Wada, Tomohito Hatakeyama, Shingo Tomita, and Tomohiro Ezura for helping with the related experiments. We sincerely thank Dr. LCA Larry, Prof. of Field Science Center, Yamagata University, for pre-reviewing our manuscript. We would like to thank Editage (www.editage.com.) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Paul Bodelier.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 6425 kb)
Rights and permissions
About this article
Cite this article
Wagatsuma, T., Tanaka, K., Iino, Y. et al. Burr marigold (Bidens tripartita L.) roots directly and immediately scavenge rhizosphere methane with highly exuded hydrogen peroxide via a rhizosphere Fenton reaction. Plant Soil 459, 289–313 (2021). https://doi.org/10.1007/s11104-020-04766-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-020-04766-z