Abstract
Fusarium wilt caused by Fusarium oxysporum f. sp. lycopersici, a hemibiotrophic filamentous fungal pathogen is one of the important diseases of tomato. Recently, RNA interference (RNAi) has emerged as a novel alternative strategy for the control of plant diseases caused by fungal pathogens. Therefore, we tested the potential of RNAi for the control of Fusarium wilt in tomato by targeting a key polyamine (PA) biosynthesis gene, ornithine decarboxylase (ODC) of the pathogen as PAs (putrescine, spermidine, and spermine) are absolutely essential for normal growth and development. The target fungal ODC gene fragment was cloned in a hairpin RNA construct, which was used to develop several transgenic tomato plants. These RNAi transgenic lines expressed the intended small interfering RNAs (siRNAs) and exhibited moderate to high resistance when challenged with the spores of F. oxysporum. Interestingly, the silencing effect was confined only to the fungal pathogen and had no influence on the plant ODC gene expression, polyamine levels, and morphology. These results confirm that the ODC gene is vital for fungal growth and is a suitable target for disease control through host-induced gene silencing (HIGS) and also implicates the transfer of dsRNA/siRNAs from host plant cells to the fungal cells. To further validate the uptake of plant-derived siRNAs by fungal cells in a visual manner, silencing of the GFP transgene was observed in F. oxysporum GFP transformants upon infecting the dsGFP-expressing transgenic tomato plants. These experiments demonstrate the applicability of RNAi-based approach for crop protection from the filamentous fungal pathogens.





Similar content being viewed by others
References
Agrawal N, Dasaradhi PV, Mohmmed A, Malhotra P, Bhatnagar RK, Mukherjee SK (2003) RNA interference: biology, mechanism, and applications. Microbiol. Mol. Biol Rev 67:657–685
Agrios GN (1988) Plant pathology, 3rd ed. academic press, Inc., New York, p 803
Andrade CM, Tinoco MLP, Rieth AF, Maia FCO, Aragao FJL (2016) Host-induced gene silencing in the necrotrophic fungal pathogen Sclerotinia sclerotiorum. Plant Pathol 65:626–632
Armas-Tizapantzi A, Montiel-Gonzalez AM (2016) RNAi silencing: a tool for functional genomics research on fungi. Fungal Biol Rev 30:91–100
Bailey A, Mueller E, Bowyer P (2000) Ornithine decarboxylase of Stagnospora (Septoria) nodorum is required for virulence toward wheat. J Biol Chem 275:14242–14247
Bajaj S, Rajam MV (1996) Polyamine accumulation and near-loss of morphogenesis in long-term callus cultures of rice: restoration of plant regeneration by manipulation of cellular polyamine levels. Plant Physiol 112:1343–1348
Bekele D, Tesfaye K, Fikre A (2019) Applications of virus induced gene silencing (VIGS) in plant functional genomics studies. J Plant Biochem Physiol 7:229
Bharti P, Rajam MV (1995) Effects of the polyamine biosynthesis inhibitor difluoromethylornithine on growth, polyamine levels, chromosome behavior and polygenic traits of wheat (Triticum aestivum L.). Ann Bot 76:297–301
Bharti P, Jyoti P, Kappoor P, Sharma V, Shanmugam V, Yadav SK (2017) Host-induced silencing of pathogenicity genes enhances resistance to Fusarium oxysporum wilt in tomato. Mol Biotechnol 59:343–352
Carreras-Villasenor N, Esquivel-Naranjo EU, Villalobos-Escobedo JM (2013) The RNAi machinery regulates growth and development in the filamentous fungus Trichoderma atroviride. Mol Microbiol 89:96–112
Chen W, Kastner C, Nowara D, Oliveira-Garcia E, Rutten T, Zhao Y, Deising HB, Kumlehn J, Schweizer P (2016) Host-induced silencing of Fusarium culmorum genes protects wheat from infection. J Exp Bot 67:4979–4991
Cheng W, Song X-S, Li H-P, Cao L-H et al (2015) Host-induced gene silencing of an essential chitin synthase gene confers durable resistance to Fusarium head blight and seedling blight in wheat. Plant Biotechnol J 13:1335–1345
Choubey A, Rajam MV (2017) Transcriptome response and developmental implications of RNA-mediated ODC knockdown in tobacco. Funct Integr Genomics 17:399–412
Cooper B, Campbell KB (2017) Protection against common bean rust conferred by a gene-silencing method. Phytopathology 107:920–927
Crespo-Sempere A, Estiarte N, Marin S, Sanchis V, Ramos AJ (2015) Targeting Fusarium graminearum control via polyamine enzyme inhibitors and polyamine analogs. Food Microbiol 49:95–103
De Coninck B, Timmermans P, Vos C, Cammue BP, Kazan K (2015) What lies beneath: belowground defense strategies in plants. Trends Plant Sci 20:91–101
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Fernandes JS, Angelo PCS, Cruz JC, Santos JMM, Sousa NR, Silva GF (2016) Post-transcriptional silencing of the SGE1 gene induced by a dsRNA hairpin in Fusarium oxysporum f. sp. cubense, the causal agent of Panama disease. Genet Mol Res 15:https://doi.org/10.4238/gmr.15027941
Getsinger KD (1998) Chemical control research in the corps of engineers. J Aquat Plant Manag 36:61–64
Ghag SB, Shekhawat UKS, Ganapathi TR (2014) Host-induced post-transcriptional hairpin RNA-mediated gene silencing of vital fungal genes confers efficient resistance against Fusarium wilt in banana. Plant Biotechnol J 12:541–553
Govindarajulu M, Epstein L, Wroblewski T, Michelmore W (2015) Host-induced gene silencing inhibits the biotrophic pathogen causing downy mildew of lettuce. Plant Biotechnol J 13:875–883
Grover A, Gowthaman R (2003) Transgenic plants for fungal resistance. Curr Sci 84:330–340
Guevara-Olvera L, Xoconostle-Cazares B, Ruiz-Herrera J (1997) Cloning and disruption of the ornithine decarboxylase gene of Ustilago maydis: evidence for a role of polyamines in its dimorphic transition. Microbiology 143:2237–2245
Hernandez I, Borras O, Chacon O, Pujol M, Lopez Y, Rodrigues R, Portieles R2 (2010) Demonstration by RNA interference of a new molecular mechanism for resistance to an oomycete in tobacco plants. Biotecnol Apl 27:242–244
Hofgen R, Willmitzer L (1988) Storage of competent cells for Agrobacterium transformation. Nucleic Acids Res 16:9877
Hu Z, Parekh U, Maruta N, Trusov Y, Botella JR (2015) Down-regulation of Fusarium oxysporum endogenous genes by host-delivered RNA interference enhances disease resistance. Front Chem 3:1. https://doi.org/10.3389/fchem.2015.00001
Ito S, Takahara H, Kawaguchi T, Tanaka S, Kameya-Iwaki M (2002) Post transcriptional silencing of the tomatinase gene in Fusarium oxysporum f. sp lycopersici. J Phytopathology 150:474–480
Jackson JL, Bartz SR, Schelter J, Kobayashi SV, Burchard J, Mao M, Li B, Cavet G, Linsley PS (2003) Expression profiling reveals off-target gene regulation by RNAi. Nat Biotechnol 21:635–637
Jiménez-Bremont JF, Ruiz-Herrera J, Dominguez A (2001) Disruption of gene YlODC reveals absolute requirement of polyamines for mycelial development in Yarrowia lipolytica. FEMS Yeast Res 1:195–204
Jiménez-Bremont JF, Rodríguez-Kessler M, Rodriguez-Guerra R, Cortes-Penagos C et al (2006) Cloning and sequence analysis of ornithine decarboxylase gene fragments from the Ascomycota. Mitochondrial DNA 17:231–236
Jones JB, Jones JP, Stall RE, Zitter TA (1991) Compendium of tomato diseases. American Phytopathology Society Press, St Paul, MN
Khan AJ, Minocha SC (1989a) Biosynthetic arginine decarboxylase in some phytopathogenic fungi. Life Sci 44:1215–1222
Khan AJ, Minocha SC (1989b) Polyamine biosynthetic enzymes and the effect of their inhibition on the growth of some phytopathogenic fungi. Plant Cell Physiol 30:655–663
Khatri M, Rajam MV (2007) Targeting polyamines of Aspergillus nidulans by siRNA specific to fungal ornithine decarboxylase gene. Med Mycol 45:211–220
Koch A, Kogel K-H (2014) New wind in the sails: improving the agronomic value of crop plants through RNAi-mediated gene silencing. Plant Biotechnol J 12:821–831
Koch A, Kumar N, Weber L, Keller H, Imani J, Kogel K-H (2013) Host-induced gene silencing of cytochrome P450 lanosterol C14a-demethylase-encoding genes confers strong resistance to Fusarium species. Proc Natl Acad Sci U S A 110:19324–19329
Koch A, Biedenkop D, Furch A, Weber L et al (2016) An RNAi-based control of Fusarium graminearum infections through spraying of long dsRNAs involves a plant passage and is controlled by the fungal silencing machinery. PLoS Pathog 12:e1005901
Kumar M (2011) RNAi-mediated targeting of acetylcholinesterase gene of Helicoverpa armigera for insect resistance in transgenic tobacco and tomato. Ph.D Thesis. University of Delhi, Delhi, India
Kumria R (2000) Modulation of polyamine biosynthesis, plant regeneration and stress responses in transgenic rice and tobacco by introduction of ornithine decarboxylase gene. Ph. D Thesis, University of Delhi, Delhi, India
Kusano T, Berberich T, Tateda C, Takahashi Y (2008) Polyamines: essential for growth and survival. Planta 228:367–381
Lagopodi AL, Ram AFL, Lamers GEM, Punt PJ, Van den Hondel CA, Lugtenberg BJ, Bloemberg GV (2002) Novel aspects of tomato root colonization and infection by Fusarium oxysporum f. sp. radicis-lycopersici revealed by confocal laser scanning microscopic analysis using the green fluorescent protein as a marker. Mol Plant-Microbe Interact 15:172–179
Lin B, Xiao Y (1995) Sources of resistance to Verticillium wilt in Solanum melongena and its affinities identified by improved root dip method. Capsicum Eggplant Newslett 14:81–84
Lu C, Meyers BC, Green PJ (2007) Construction of small RNA cDNA libraries for deep sequencing. Methods 43:110–117
Madhulatha P, Pandey R, Hazarika P, Rajam MV (2007) High transformation frequency in Agrobacterium-mediated genetic transformation of tomato by using polyamines and maltose in shoot regeneration medium. Physiol Mol Biol Plants 13:191–198
Mamta B, Rajam MV (2017) RNAi technology: a new platform for crop pest control. Physiol Mol Biol Plants 23:487–501
McGovern RJ (2015) Management of tomato diseases caused by Fusarium oxysporum. Crop Prot 73:78–92
Mcloughlin AG, Walker PL, Wytinck N, Sullivan DS, Whyard S, Belmonte MF (2018) Developing new RNA interference technologies to control fungal pathogens. Can J Plant Pathol 40:325–335
Nowara D, Gay A, Lacomme C, Shaw J, Ridout C, Douchkov D, Hensel G, Kumlehn J, Schweizer P (2010) HIGS: host-induced gene silencing in the obligate biotrophic fungal pathogen Blumeria graminis. Plant Cell 22:3130–3141
Pareek M, Rajam MV (2017) RNAi-mediated silencing of MAP kinase signaling genes (Fmk1, Hog1, and Pbs2) in Fusarium oxysporum reduces pathogenesis on tomato plants. Fungal Biol 121:775–784
Puyam A, Sharma S, Kashyap PL (2017) RNA interference: a novel approach for plant disease management. J Appl Nat Sci 9:1612–1618
Qi T, Guo J, Peng H, Liu P, Kang Z, Guo J (2019) Host-induced gene silencing: a powerful strategy to control diseases of wheat and barley. Int J Mol Sci 20:206. https://doi.org/10.3390/ijms20010206
Rajam MV (1993) Polyamine biosynthesis inhibitors: new protectants against fungal diseases. Curr Sci 65:461–469
Rajam MV (1998) Polyamine biosynthetic pathway: a potential target for plant chemotherapy. Curr Sci 74:729–731
Rajam MV (2005) Prevention of plant diseases by targeting fungal polyamine biosynthesis. In: Satyanarayana T, Johri BN (eds) microbial diversity: current perspectives and potential applications, New Delhi, I.K. international Pvt. ltd, pp 457–470
Rajam MV (2012) Host induced silencing of fungal pathogen genes: an emerging strategy for disease control in crop plants. Cell Dev Biol 1:1–2. https://doi.org/10.4172/2168-9296.1000e118
Rajam MV, Galston AW (1985) The effects of some polyamine biosynthetic inhibitors on growth and morphology of pathogenic fungi. Plant Cell Physiol 26:683–692
Rajam MV, Weinstein LH, Galston AW (1985) Prevention of a plant disease by specific inhibition of fungal polyamine biosynthesis. Proc Natl Acad Sci U S A 82:6874–6878
Rajam MV, Weinstein LH, Galston AW (1989) Inhibition of uredospore germination and germ tube growth by inhibitors of polyamine metabolism in Uromyces phaseoli L. Plant Cell Physiol 30:349–350
Reiz M, Walters D, Moerschbacher B (1995) Germination and appressorial formation by uredospores of Uromyces viciae-fabae exposed to inhibitors of polyamine. Eur J Plant Pathol 101:573–578
Rosa C, Kuo Y-W, Wuriyanghan H, Falk BW (2018) RNA interference mechanisms and applications in plant pathology. Annu Rev Phytopathol 56:581–610
Schumann U, Smith NA, Kazan K, Ayliffe M, Wang M-B (2013) Analysis of hairpin RNA transgene-induced gene silencing in Fusarium oxysporum. Silence 4:3
Singh N (2011) Genetic engineering of tomato for Fusarium wilt resistance by in plant RNAi- mediated silencing of fungal ornithine decarboxylase gene. Ph.D Thesis. University of Delhi, Delhi, India
Steinkellner S, Mammerler R, Vierheilig H (2005) Microconidia germination of the tomato pathogen Fusarium oxysporum in the presence of root exudates. J Plant Interact 1:23–30
Tetorya M, Rajam MV (2018) RNA silencing of PEX6 gene causes decrease in pigmentation, sporulation and pathogenicity of Fusarium oxysporum. Plant Pathol 67:67–75
Tinoco MLP, Dias BBA, Dall’Astta RC, Pamphile JA, Aragao FJL (2010) In vivo trans-specific gene silencing in fungal cells by in planta expression of a double-stranded RNA. BMC Biol 8:27–37
Tomilov AA, Tomilova NB, Wroblewski T, Michelmore R, Yoder JI (2008) Trans-specific gene silencing between host and parasitic plants. Plant J 56:389–397
Trieu TA, Navarro-Mendoza MI, Perex-Arques CP, Sanchis M et al (2017) RNAi-based functional genomics identifies new virulence determinants in Mucormycosis. PLoS Pathog 13:e1006150
Valdes-Santiago L, Cervantes-Chavez JA, Leon-Ramirez CG, Ruiz-Herrera J (2012) Polyamine metabolism in fungi with emphasis on phytopathogenic species. J Amino Acids 2012:1–13
Wally O, Punja ZK (2010) Genetic engineering for increasing fungal and bacterial disease resistance in crop plants. GM Crops 1:199–206
Walters DR (1995) Inhibition of polyamine biosynthesis in fungi. Mycol Res 99:129–139
Wang M, Weiberg A, Lin F-M, Thomma BPHJ, Huang H-D, Jin H (2016) Bidirectional cross-kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nat Plants 2: https://doi.org/10.1038/nplants.2016.151
Yin C, Jurgenson JE, Hulbert SC (2011) Development of a host-induced RNAi system in the wheat stripe rust fungus Puccinia striiformis f. sp. tritici. Mol Plant-Microbe Interact 24:554–561
Yogindran S, Rajam MV (2015) RNAi for crop improvement. In: Bahadur B et al (eds) Plant biotechnology: volume II: plant genomics and biotechnology. Springer India, New Delhi, pp 623–637
Zhang T, Zhao Y-L, Zhao J-H, Wang S, Jin Y, Chen ZQ, Fang YY, Hua CL, Ding SW, Guo HS (2016) Cotton plants export microRNAs to inhibit virulence gene expression in a fungal pathogen. Nat Plants 2:16153. https://doi.org/10.1038/nplants.2016.153
Zhou B, Bailey A, Niblett CL, Qu R (2016) Control of brown patch (Rhizoctonia solani) in tall fescue (Festuca arundinaceae Schreb.) by host induced gene silencing. Plant Cell Rep 35:791–802
Acknowledgments
NS acknowledges the Council of Scientific and Industrial Research for senior research fellowship. We also thank the University Grants Commission, New Delhi, for special assistance program (DRS-III); Department of Science and Technology (DST), New Delhi, for FIST program (Level 2); and DU-DST PURSE grant.
Funding
We are grateful to the Department of Biotechnology (Grant No. BT/PR 10713/AGR/36/601/2008), New Delhi, for the financial assistance (to MVR).
Author information
Authors and Affiliations
Contributions
MVR has conceived the concept and designed the experiments. NS performed the experiments, generated and analyzed the data, and written the manuscript. MVR and SKM revised the manuscript and interpreted the data. All the authors approved the final manuscripts.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Message
• Silenced ornithine decarboxylase (ODC) gene of Fusarium oxysporum f. sp. lycopersici by host-induced RNAi
• Tomato RNAi lines have exhibited Fusarium wilt resistance.
• Validated uptake of plant-derived siRNAs by fungi using GFP gene
• Demonstrated that ODC gene is vital for fungal development and good target for plant fungal infections through RNAi
Electronic Supplementary Material
ESM. 1
(DOCX 12 kb)
Fig. S1
Pathway for biosynthesis of major plant and fungal polyamines. Initial step in the pathway involves formation of diamine, putrescine from ornithine decrboxylation by ODC enzyme activity in all the organisms including, fungi. α-Difluoromethylornithine (DFMO) irreversibly inhibits ODC activity their by blocking putrescine synthesis. Alternatively, putrescine is also produced from arginine by the activity of ADC enzyme in plants, bacteria and some fungi. Higher polyamines, triamine spermidine and tetraamine spermine are synthesized from putrescine by the catalytic activity of enzymes spermidine synthase (SPDSYN) and spermine synthase (SPMSYN), respectively, by sequential additions of aminopropyl groups donated by decarboxylated S- adenosylmethionine (dcSAM), which is in turn synthesized from SAM by SAM decarboxylase. (JPG 41 kb)
Fig. S2
Diagrammatic representation of pFGC5941 RNAi vector. It has basta resistance (bar) gene for plant selection, CaMV35S promoter to drive the dsRNA expression, ChsA intron to stabilize the dsRNA molecules hairpin formation. The 541 bp ODC gene sequence was inserted in the vector in sense and antisense orientation with AscI, SwaI and BamHI, XbaI restriction sites, respectively (a) and this complete expression cassette was cleaved with EcoRI and PstI restriction enzymes and inserted into pCambia 2300 binary vector, which has NPT-II as the selection marker (b). (JPG 47 kb)
Fig. S3
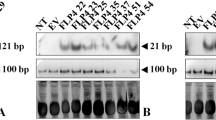
Transgene integration in ODC RNAi tomato plants. The presence of transgenes was checked by PCR analysis using both ODC and NPT-II gene primers, Lane 1 (of a, b, c, d) is marker (M, 1 kb DNA ladder) and lane 2 is +ve control (plasmid DNA), Lane 3–11 (a), 2–9 (b) are the transgenic lines showing ODC gene integration and Lane 2, 3 (c) are the untransformed control and + ve control respectively, lanes 4–11 (c); lane 2–13 (d) are the transgenic lines showing NPT-II gene integration. (JPG 61 kb)
Fig. S4
The expression of native ODC and ADC genes in ODC hp. tomato transgenic plants by RT- PCR with tomato ODC and ADC primers. Lane 1 (of a, b, c, d) is the marker (1 kb DNA ladder); Lane 2 (a, b, c, d) are the wild-type control and Lanes 3–10 (a, ODC), 3–7 (c, ADC) represent the transgenic lines. The β- actin RT-PCR product was taken as the internal control in this experiment (b, d). This experiment was repeated thrice. (JPG 58 kb)
Fig. S5
(a) The diagrammatic representation of pCAMBIA 1302 vector carrying sGFP gene used for F. oxysporum transformation; (b) Diagrammatic representation of GFP hp. construct used for tomato transformation (JPG 43 kb)
Rights and permissions
About this article
Cite this article
Singh, N., Mukherjee, S.K. & Rajam, M.V. Silencing of the Ornithine Decarboxylase Gene of Fusarium oxysporum f. sp. lycopersici by Host-Induced RNAi Confers Resistance to Fusarium Wilt in Tomato. Plant Mol Biol Rep 38, 419–429 (2020). https://doi.org/10.1007/s11105-020-01205-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-020-01205-2