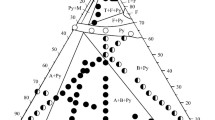
Phase equilibria and structural transformations in the ternary ZrO2–La2O3–Gd2O3 system at 1600°C were studied by X-ray diffraction, microstructural, and electron microprobe analyses over the entire composition range. Fields of solid solutions based on the cubic fluorite-type (F) and tetragonal (T) modifications of ZrO2, monoclinic (M) and cubic (C) modifications of Gd2O3, hexagonal (A) modification of La2O3, and an ordered intermediate phase with La2Zr2O7 pyrochloretype structure (Py) exist in the system. The boundaries of phase fields and lattice parameters of the phases were determined. In the ZrO2-rich corner, solid solutions based on the ZrO2 tetragonal modification are formed. The solubility of La2O3 in the T-ZrO2 lattice is low and reaches ~0.5 mol.%, which is evidenced by X-ray diffraction and microstructural analyses. The solid solutions based on the tetragonal modification of zirconia cannot be quenched when cooled with the furnace. The diffraction patterns recorded at room temperature include peaks of the M-ZrO2 monoclinic phase. The ordered Ln2Zr2O7 pyrochlore-type (Py) phase is in equilibrium with all phases (except for C-Gd2O3) existing in the ternary ZrO2–La2O3–Gd2O3 system at 1600°C and forms substitutional solid solutions with phases of the binary systems. The greatest solubility in the Ln2Zr2O7 (Py) lattice is shown by Gd2O3 along the Gd2O3–(67 mol.% ZrO2–33 mol.% La2O3) section. The La2Zr2O7 (Py) lattice parameters change from a = 1.0781 nm for the single-phase (Py) sample containing 67 mol.% ZrO2–33 mol.% La2O3–0 mol.% Gd2O3 to a = 1.0741 nm for the threephase (F + Py + B) sample containing 43.55 mol.% ZrO2–21.45 mol.% La2O3–35 mol.% Gd2O3 along the Gd2O3–(67 mol.% ZrO2–33 mol.% La2O3) section. The cubic phases are primarily in equilibrium in the ZrO2–La2O3–Gd2O3 system: F-Fm3m, Py-Fd3m, and C-Ia3. The isothermal section of the ZrO2–La2O3–Gd2O3 phase diagram at 1600°C contains four three-phase regions (T + F + Py, A + Py + B, Py + F + B, and F + C + B) and nine two-phase regions (T + Py, F + Py, F + T, F + C, F + B, B + Py, A + Py, B + A, and B + C).






Similar content being viewed by others
References
V.A. Vorozhtcov, V.L. Stolyarova, S.I. Lopatin, E.P. Simonenko, N.P. Simonenko, K.A. Sakharov, V.G. Sevastyanov, and N.T. Kuznetsov, “Vaporization and thermodynamic properties of lanthanum hafnate,” J. Alloys Compd., 735, 2348–2355 (2018).
Kwon Chang-Sup, Lee Sung-Min, Oh Yoon-Suk, Kim Hyung-Tae, Jang Byung-Koog, and Kim Seongwon, “Preparation of suspension in La2O3–Gd2O3–ZrO2 system via planetary mill and characteristics of (La1–xGdx)2Zr2O7 coatings fabricated via suspension plasma spray,” J. Korean Powder Metall. Inst., 20, No. 6 (2013).
Kwon Chang-Sup, Sung-Min Lee, Yoon-Suk Oh, Hyung-Tae Kim, Byung-Koog Jang, and Seongwon Kim, “Phase evolution and thermal conductivities of (La1–xGdx)2Zr2O7 oxides for thermal barrier coatings,” J. Korean Powder Metall. Inst., 19, 429–434 (2012).
Kwon Chang-Sup, Sung-Min Lee, Yoon-Suk Oh, Hyung-Tae Kim, Byung-Koog Jang, and Seongwon Kim, “Structure and thermal conductivity of thermal barrier coatings in lanthanum/gadolinium zirconate system fabricated via suspension plasma spray,” J. Korean Inst. Surf. Eng., 47, 316–322 (2014).
S.A. Toropov, Phase Diagrams of Refractory Oxide Systems [in Russian], Nauka, Leningrad (1987).
M. Zinkevich, “Thermodynamics of earth sesquioxedes,” Prog. Mater. Sci., 52, 597–647 (2007).
Yumin Zhang, Thermodynamic Properties of Rare Earth Sesquioxide, McGill University, Montreal, QC, Canada (2016).
J. P. Coutures and M. Foex, “High-temperature study of systems formed by lanthanum sesquioxide and lanthanide sesquioxides. I. Phase diagrams (1400°C < T < T liquidus),” J. Solid State Chem., 182, No. 17, 171–182 (1976).
R. Hory’n, E. Bukowska, and A. Sikora, “Phase relations in La2O3–Gd2O3–CuO system at 950°C,” J. Alloys Compd., 416, 209–213 (2006).
S.J. Schneider and R.S. Roth, “Phase equilibria in systems involving the rare-earth oxides. Part II. Solid state reactions in trivalent rare-earth oxide systems,” J. Res. Nat. Bur. Stand. A. Phys. Chem., 64A, No. 4, 317–332 (1960).
E.R. Andrievskaya, O.A. Kornienko, and A.I. Bykov, “Interaction of lanthanum and gadolinium oxides at 1100°C,” Sovr. Probl. Fiz. Materialoved., No. 26, 23–30 (2017).
A. Rouanet, “Contribution to the study of zirconium oxide–lanthanide oxide systems near the melting point: Theses,” Rev. Int. High Temp. Refract., 8, No. 2, 161–180 (1971).
Ch. Wang, M. Zinkevich, O. Fabrichnaya, and F. Aldinger, “Experimental investigation and thermodynamic modeling in the ZrO2–GdO1.5 system,” in: Calphad XXXIII Program and Abstracts (2004), p. 88.
V. Grover and A.K. Tyagi, “Phase relations studies in the CeO2–Gd2O3–ZrO2 system,” J. Solid State Chem., 177, 4197–4204 (2004).
Ch. Wang, M. Zinkevich, and F. Aldinger, “Phase diagrams and thermodynamics of rare-earth-doped zirconia ceramics,” Pure Appl. Chem., 79, No. 10, 1731–1753 (2007).
A. Negro and I. Amato, “An investigation of the zirconia-gadolinia system,” J. Less-Common Met., 2, No. 1, 81–88 (1972).
T. Van Dijk, K.J. De Vries, and F.J. Burggaf, “Electrical conductivity of fluorite and pyrochlore LnxZr1–xO2–x/2 (Ln = Gd, Nd) solid solutions,” Phys. Status Solidi, 58(a), 115–125 (1980).
A.J. Feighery, J.T. Zheng, and S.C. Irvine, “Phase relations at 1500°C in the ternary system ZrO2–Gd2O3–TiO2,” J. Solid State Chem., 160, 302–306 (2001).
E.R. Andrievskaya and O. A. Kornienko, “Interaction between gadolinium oxide and zirconium oxide at 1500°C,” Sbor. Nauch. Tr. OAO UkrNII Ogneup. Berezhny, No. 109, 117–125 (2009).
E.R. Andrievskaya and L.M. Lopato, “Approximating the liquidus surface of the ZrO2–Y2O3–La2O3 phase equilibrium diagram with reduced polynomials,” Powder Metall. Met. Ceram., 39, No. 9–10, 445–450 (2000).
B. Bastide, P. Odier, and J.P. Coutures, “Phase equilibrium and martensitic transformation in lanthana–doped zirconia,” J. Am. Ceram. Soc., 71, No. 6, 449–453 (1988).
Author information
Authors and Affiliations
Corresponding author
Additional information
E.R. Andrievskaya is deceased
Translated from Poroshkova Metallurgiya, Vol. 58, Nos. 11–12 (530), pp. 119–131, 2019.
Rights and permissions
About this article
Cite this article
Andrievskaya, E., Kornienko, O., Bykov, A. et al. Phase Equilibria in the ZrO2–La2O3–Gd2O3 System at 1600°C. Powder Metall Met Ceram 58, 714–724 (2020). https://doi.org/10.1007/s11106-020-00128-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-020-00128-7