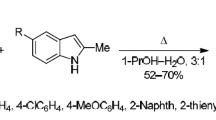
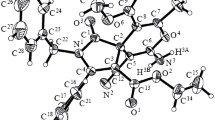
Abstract
Base-catalyzed rearrangements of both individual 4-(acylmethylidene)butenolides and their mixtures prepared by condensation of citraconic anhydride with various phosphoranes occur successfully only in the presence of 5.2% MeONa in MeOH (molar ratio MeONa: substrate ≤ 10: 1, room temperature, 1–2 h). Under these conditions, the yields of 2-cinnamoyl-4-methylcyclopent-4-ene-1,3-dione (coruscanone B) and 2-acetyl-4-methylcyclopent-4-ene-1,3-dione are 56 and 65%, respectively. With a considerable increase in the reaction temperature or the molar ratio MeONa: substrate, formal addition of MeOH to the C(4)=C(5) double bond of these triketones becomes an appreciable (or predominant) process. A reaction of coruscanone B with CH2N2 in ether gives coruscanone A as a ~3: 2 mixture of (Z)- and (E)-methyl enolates (43%); other products (10%) result from the expansion and aromatization of the five-membered ring of the triketone. The simplest analog of coruscanone B, 2-acetyl-4-methylcyclopent-4-ene-1,3-dione, reacts with CH2N2 in a similar way.
Similar content being viewed by others
References
X.-C. Li, D. Ferreira, M. R. Jacob, Q. Zhang, S. I. Khan, H. N. ElSohly, D.G. Nagle, T. J. Smillie, I. A. Khan, L. A. Walker, A. M. Clark, J. Am. Chem. Soc., 2004, 126, 6872.
K. S. Babu, X.-C. Li, M. R. Jacob, Q. Zhang, S. I. Khan, D. Ferreira, A. M. Clark, J. Med. Chem., 2006, 49, 7877.
E. I. Hwang, Y. M. Lee, S. M. Lee, W. H. Yeo, J. S. Moon, T. H. Kang, K. D. Park, S. U. Kim, Planta Med., 2007, 73, 679.
H. M. Oh, S. K. Choi, J. M. Lee, S. K. Lee, H. Y. Kim, D. C. Han, H. M. Kim, K. H. Son, B. M. Kwon, Bioorg. Med. Chem., 2005, 13, 6182.
S. Y. Wang, X. Y. Lan, J. H. Xiao, J. C. Yang, Y. T. Kao, S. T. Chang, Phytother. Res., 2008, 22, 213.
S. Kumar, K. Jayabal, S. Y. Wang, Planta Med., 2008, 74, 956.
Y. Aoyama, T. Konoike, A. Kanda, N. Naya, M. Nakajima, Bioorg. Med. Chem. Lett., 2001, 11, 1695.
O. P. Shestak, Ph.D. (Chem.) Thesis, Tikhookeansk. Inst. Bioorg. Khim. DVO RAN, Vladivostok, 1987, 210 pp. (in Russian).
O. P. Shestak, V. L. Novikov, S. I. Stekhova, I. A. Gorsh-kova, Khim.-Farm. Zh., 1999, 33, 1, 18 [Pharm. Chem. J. (Engl. Transl.), 1999, 33, No. 1, 18].
O. P. Shestak, V. L. Novikov, N. G. Prokof’eva, E. L. Chaikina, Khim.-Farm. Zh., 1999, 33, 12, 5 [Pharm. Chem. J. (Engl. Transl.), 1999, 33, 631].
V. L. Novikov, O. P. Shestak, V. V. Logachev, M. M. Anisimov, Rastitel’nye resursy [Plant Resources], 2003, 39, No. 4, 87 (in Russian).
L. A. Lapshina, O. P. Shestak, A. V. Reunov, V. L. Novikov, M. M. Anisimov, Rastitel’nye resursy [Plant Resources], 2006, 44, No. 1, 107 (in Russian).
O. P. Shestak, E. A. Martyyas, M. M. Anisimov, V. L. No-vikov, Tezisy dokladov Mezhdunarodnoi nauchnoi konferentsii “Khimiya, tekhnologiya i meditsinskie aspekty prirodnykh soedinenii” (Almaty, Kazakhstan, 10–13 oktyabrya 2007 g.) [Abstrs Int. Scientific Conf. “Chemistry, Technology, and Medicinal Aspects of Natural Compounds” (Almaty, Kazakhstan, October 10–13, 2007)], Almaty, 2007, p. 250 (in Russian).
L. C. Diaz, S. B. Shimokomaki, R. T. Shiota, J. Braz. Chem. Soc., 2005, 16, 482.
D. R. Gedge, G. Pattenden, J. Chem. Soc., Chem. Commun., 1978, 20, 880.
N. G. Clemo, D. R. Gedge, G. Pattenden, J. Chem. Soc., Perkin Trans. 1, 1981, 5, 1448.
V. A. Facundo, A. L. Sa, S. A. Silva, S. M. Morais, C. R. Matos, R. Braz-Filho, J. Braz. Chem. Soc., 2004, 15, 140.
D. V. Berdyshev, V. P. Glazunov, V. L. Novikov, Tezisy dokladov VI Vserossiiskogo nauchnogo seminara “Khimiya i meditsina” (Ufa, 26–29 noyabrya 2007 g.) [Abstrs, VI All-Russia Scientific Seminar “Chemistry and Medicine” (Ufa, November 26–29, 2007)], Ufa, Gilem, 2007, P–133 (in Russian).
C. Reichardt, Losungsmittel — Effekte in der organischen Chemie, Verlag Chemie, 1969.
A. K. Kiang, H. H. Lee, K. Y. Sim, J. Chem. Soc., 1962, 11, 4338.
H. H. Lee, C. H. Tan, J. Chem. Soc. (C), 1967, 17, 1583.
A. A. Akhrem, A. M. Moiseenkov, F. A. Lakhvich, A. I. Poselenov, T. M. Ivanova, Izv. Akad. Nauk SSSR, Ser. Khim., 1971, 371 [Bull. Acad. Sci. USSR, Div. Chem. Sci. (Engl. Transl.), 1971, 20, 305].
A. A. Akhrem, A. M. Moiseenkov, F. A. Lakhvich, A. I. Poselenov, Izv. Akad. Nauk SSSR, Ser. Khim., 1972, 143 [Bull. Acad. Sci. USSR, Div. Chem. Sci. (Engl. Transl.), 1972, 21, 128].
V. L. Novikov, O. P. Shestak, N. N. Balaneva, Ya. Ya. Paulin’sh, G. B. Elyakov, Izv. Akad. Nauk Latv. SSR, Ser. Khim., 1985, 6, 718 (in Russian).
F. Ramirez, S. Dershowitz, J. Org. Chem., 1957, 22, 41.
S. F. Ingham, R. A. Massy-Westropp, G. D. Reynolds, Aust. J. Chem., 1974, 27, 1477.
C. F. Ingham, R. A. Massy-Westropp, G. D. Reynolds, W. D. Thorpe, Aust. J. Chem., 1975, 28, 2499.
M. Nilsson, Acta Chem. Scand., Ser. A, 1964, 18, 441.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 1, pp. 82–91, January, 2010.
Rights and permissions
About this article
Cite this article
Shestak, O.P., Novikov, V.L. Synthesis of coruscanones A and B, metabolites of Piper coruscans, and related compounds. Russ Chem Bull 59, 81–90 (2010). https://doi.org/10.1007/s11172-010-0048-9
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-010-0048-9