Abstract
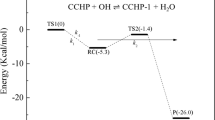
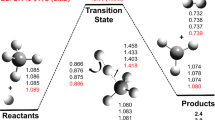
In this work, the kinetics of reaction class of hydrogen abstraction from saturated hydrocarbons by O2 molecules has been studied. The high-pressure reaction rate constants were determined using reaction class transition state theory/structure-activity relationship (RC-TST/SAR) methodology, augmented by linear energy relationship (LER) and/or barrier height grouping (BHG) approximations for evaluation of the reaction barrier heights. The parameters needed have been derived from DFT calculations at M06-2X/aug-cc-pVTZ level for a training set of 23 reactions, involving hydrogen abstraction by O2 molecule at primary, secondary, and tertiary carbon sites. The reference reaction rate constant C2H6 + O2 → C2H5 + HO2 was obtained by extrapolation of the simplest reaction within the title family CH4 + O2 → CH3 + HO2. Kinetic parameters of the later one, calculated from canonical variational transition state theory (CVT), were taken from literature. The influence of low-frequency internal rotations has been investigated in details. The error analysis shows that the average systematic error of RC-TST/SAR-derived rate constants at low temperatures is within 25% compared to the explicit RC-TST results and diminishes at higher temperatures. This suggests that the proposed methodology can be effectively implemented in the automated mechanism generation codes to create the fuel combustion mechanisms.










Similar content being viewed by others
References
Battin-Leclerc F, Curran H, Faravelli T, Glaude PA (2013) Specificities related to detailed kinetic models for the combustion of oxygenated fuels components. Chapter 4. In: Battin-Leclerc F, Simmie JM, Edward Blurock E (eds) Cleaner Combustion: Developing Detailed Chemical Kinetic Models. Springer-Verlag, London, pp 93–109
Aranda C, Richaud A, Méndez F, Domínguez A (2018) Theoretical rate constant of methane oxidation from the conventional transition-state theory. J Mol Model 24(10):294. https://doi.org/10.1007/s00894-018-3829-y
Srinivasan NK, Michael JV, Harding LB, Klippenstein SJ (2007) Experimental and theoretical rate constants for CH4 + O2 → CH3 + HO2. Combust Flame 149(1):104–111. https://doi.org/10.1016/j.combustflame.2006.12.010
Branko R, Reinhardt EP, von Gregor L, Deepti K, Alexander B, David L, David M, Albert FW (2005) Active thermochemical tables: thermochemistry for the 21st century. J Phys Conf Ser 16(1):561
Mai TV-T, Duong M, Le XT, Huynh LK, Ratkiewicz A (2014) Direct ab initio dynamics calculations of thermal rate constants for the CH4 + O2 = CH3 + HO2 reaction. Struct Chem 25(5):1495–1503
Jasper AW, Klippenstein SJ, Harding LB (2009) Theoretical rate coefficients for the reaction of methyl radical with hydroperoxyl radical and for methylhydroperoxide decomposition. Proc Combust Inst 32(1):279–286. https://doi.org/10.1016/j.proci.2008.05.036
Pelevkin AV, Sharipov AS (2019) Interaction of CH4 with electronically excited O2: ab initio potential energy surfaces and reaction kinetics. Plasma Chem Plasma Process. https://doi.org/10.1007/s11090-019-10008-7
Falconer WE, Knox JH, Trotman-Dickenson AF (1961) Competitive oxidations. Part II. The lower alkanes and cyclopropane. J Chem Soc:782–791
Pelucchi M, Cavallotti C, Faravelli T, Klippenstein SJ (2018) H-Abstraction reactions by OH, HO2, O, O2 and benzyl radical addition to O2 and their implications for kinetic modelling of toluene oxidation. Phys Chem Chem Phys 20(16):10607–10627. https://doi.org/10.1039/C7CP07779C
Mai TV-T, Le XT, Huynh LK (2015) Mechanism and kinetics of low-temperature oxidation of a biodiesel surrogate−methyl acetate radicals with molecular oxygen. Struct Chem 26(2):431–444. https://doi.org/10.1007/s11224-014-0495-2
Shayan K, Vahedpour M (2013) Computational mechanistic study of methanol and molecular oxygen reaction on the triplet and singlet potential energy surfaces. Struct Chem 24(4):1051–1062. https://doi.org/10.1007/s11224-012-0128-6
Carstensen H-H, Dean AM (2009) Rate constant rules for the automated generation of gas-phase reaction mechanisms. J Phys Chem A 113(2):367–380. https://doi.org/10.1021/jp804939v
Lai L, Khanniche S, Green WH (2019) Thermochemistry and group additivity values for fused two-ring species and radicals. J Phys Chem A 123(15):3418–3428. https://doi.org/10.1021/acs.jpca.9b01065
Sabbe MK, De Vleeschouwer F, Reyniers M-F, Waroquier M, Marin GB (2008) First principles based group additive values for the gas phase standard entropy and heat capacity of hydrocarbons and hydrocarbon radicals. J Phys Chem A 112(47):12235–12251. https://doi.org/10.1021/jp807526n
Wang H, Xu R, Wang K, Bowman CT, Hanson RK, Davidson DF, Brezinsky K, Egolfopoulos FN (2018) A physics-based approach to modeling real-fuel combustion chemistry - I. Evidence from experiments, and thermodynamic, chemical kinetic and statistical considerations. Combust Flame 193:502–519. https://doi.org/10.1016/j.combustflame.2018.03.019
Ratkiewicz A, Huynh LK, Truong TN (2016) Performance of first-principles-based reaction class transition state theory. J Phys Chem B 120(8):1871–1884. https://doi.org/10.1021/acs.jpcb.5b09564
Bao JL, Truhlar DG (2017) Variational transition state theory: theoretical framework and recent developments. Chem Soc Rev 46(24):7548–7596. https://doi.org/10.1039/C7CS00602K
Gao CW, Allen JW, Green WH, West RH (2016) Reaction mechanism generator: automatic construction of chemical kinetic mechanisms. Comput Phys Commun 203:212–225. https://doi.org/10.1016/j.cpc.2016.02.013
Duong MV, Nguyen HT, Truong N, Le Thong N-M, Huynh LK (2015) Multi-species multi-channel (MSMC): an ab initio-based parallel thermodynamic and kinetic code for complex chemical systems. Int J Chem Kinet 47(9):564–575. https://doi.org/10.1002/kin.20930
Ratkiewicz A, Truong TN (2006) Automated mechanism generation: from symbolic calculation to complex chemistry. Int J Quantum Chem 106(1):244–255. https://doi.org/10.1002/qua.20748
Ratkiewicz A, Truong TN (2003) Application of chemical graph theory for automated mechanism generation. J Chem Inf Comput Sci 43(1):36–44. https://doi.org/10.1021/ci020297f
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL (2009) Gaussian 09 Rev. E. Wallingford, CT
Becke AD (1993) A new mixing of Hartree–Fock and local density-functional theories. J Chem Phys 98(2):1372–1377. https://doi.org/10.1063/1.464304
Dunning TH (1989) Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J Chem Phys 90(2):1007–1023. https://doi.org/10.1063/1.456153
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Accounts 120(1):215–241. https://doi.org/10.1007/s00214-007-0310-x
Shi S (2018) Advances in modeling hydrocarbon cracking kinetic predictions by quantum chemical theory: A review. Int J Energy Res 42(10):3164–3181. https://doi.org/10.1002/er.4049
Van de Vijver R, Vandewiele NM, Bhoorasingh PL, Slakman BL, Seyedzadeh Khanshan F, Carstensen H-H, Reyniers M-F, Marin GB, West RH, Van Geem KM (2015) Automatic mechanism and kinetic model generation for gas- and solution-phase processes: a perspective on best practices, recent advances, and future challenges. Int J Chem Kinet 47(4):199–231. https://doi.org/10.1002/kin.20902
Sumathi R, Green Jr WH (2002) A priori rate constants for kinetic modeling. Theor Chem Accounts 108(4):187–213. https://doi.org/10.1007/s00214-002-0368-4
De Oliveira LP, Hudebine D, Guillaume D, Verstraete JJ (2016) A review of kinetic modeling methodologies for complex processes. Oil Gas Sci Technol 71(3). https://doi.org/10.2516/ogst/2016011
Tsang W, Hampson RF (1986) Chemical kinetic data base for combustion chemistry. Part I. Methane and Related Compounds. J Phys Chem Ref Data 15(3):1087–1279. https://doi.org/10.1063/1.555759
Baulch DL, Cobos CJ, Cox RA, Esser C, Frank P, Just T, Kerr JA, Pilling MJ, Troe J, Walker RW, Warnatz J (1992) Evaluated kinetic data for combustion modelling. J Phys Chem Ref Data 21(3):411–734. https://doi.org/10.1063/1.555908
Taylor JE, Kulich DM (1973) Homogeneous gas-phase pyrolyses with a wall-less reactor. III. The oxygen–ethane reaction. A double reversal in oxygen and surface effects. Int J Chem Kinet 5(3):455–468. https://doi.org/10.1002/kin.550050314
Fernández-Ramos A, Ellingson BA, Meana-Pañeda R, Marques JMC, Truhlar DG (2007) Symmetry numbers and chemical reaction rates. Theor Chem Accounts 118(4):813–826. https://doi.org/10.1007/s00214-007-0328-0
Truong TN (2000) Reaction class transition state theory: hydrogen abstraction reactions by hydrogen atoms as test cases. J Chem Phys 113(12):4957–4964. https://doi.org/10.1063/1.1287839
East ALL, Radom L (1997) Ab initio statistical thermodynamical models for the computation of third-law entropies. J Chem Phys 106(16):6655–6674. https://doi.org/10.1063/1.473958
Kilpatrick JE, Pitzer KS (1949) Energy levels and thermodynamic functions for molecules with internal rotation. III. Compound Rotation. J Chem Phys 17(11):1064–1075. https://doi.org/10.1063/1.1747114
Tsang W (1988) Chemical kinetic data base for combustion chemistry. Part 3: Propane. J Phys Chem Ref Data 17(2):887–951. https://doi.org/10.1063/1.555806
Tsang W (1990) Chemical kinetic data base for combustion chemistry part 4. Isobutane. J Phys Chem Ref Data 19(1):1–68. https://doi.org/10.1063/1.555877
Mehl M, Pitz WJ, Westbrook CK, Curran HJ (2011) Kinetic modeling of gasoline surrogate components and mixtures under engine conditions. Proc Combust Inst 33(1):193–200. https://doi.org/10.1016/j.proci.2010.05.027
El Marrouni K, Abou-Rachid H, Kaliaguine S (2004) Density functional theory kinetic assessment of hydrogen abstraction from hydrocarbons by O2. J Mol Struct THEOCHEM 681(1):89–98. https://doi.org/10.1016/j.theochem.2004.04.037
Ratkiewicz A, Bieniewska J, Truong TN (2011) Kinetics of the hydrogen abstraction R − OH + H → R• − OH + H2 reaction class: an application of the reaction class transition state theory. Int J Chem Kinet 43(2):78–98. https://doi.org/10.1002/kin.20531
Muszyńska M, Ratkiewicz A, Huynh LK, Truong TN (2009) Kinetics of the hydrogen abstraction C2H3• + alkane → C2H4 + alkyl radical reaction class. J Phys Chem A 113(29):8327–8336. https://doi.org/10.1021/jp903762x
Oehlschlaeger MA, Davidson DF, Hanson RK (2006) Investigation of the reaction of toluene with molecular oxygen in shock-heated gases. Combust Flame 147(3):195–208. https://doi.org/10.1016/j.combustflame.2006.08.006
Eng RA, Fittschen C, Gebert A, Hibomvschi P, Hippler H, Unterreiner AN (1998) Kinetic investigations of the reactions of toluene and of p-xylene with molecular oxygen between 1050 and 1400 K. Symp Combust 27(1):211–218. https://doi.org/10.1016/S0082-0784(98)80407-4
Baulch DL, Cobos CJ, Cox RA, Frank P, Hayman G, Just T, Kerr JA, Murrells T, Pilling MJ, Troe J, Walker RW, Warnatz J (1994) Evaluated kinetic data for combustion modeling. Supplement I. J Phys Chem Ref Data 23(6):847–848. https://doi.org/10.1063/1.555953
Acknowledgments
The authors would like to thank the Computational Center of the University of Bialystok (Grant GO-008) for providing access to the supercomputer resources and the GAUSSIAN 09 program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict(s) of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
Tables: List of processes selected to the representative (training) set for the title reaction class. The optimized geometries and frequencies of all species calculated at M06-2X/aug-cc-pVTZ level of theory for representative set. Numerical data (RC-TST/SAR-LER rate constants) for Figs. 7 and 10(a–-b). Figures: Optimized geometries of all species calculated at M06-2X/aug-cc-pVTZ level of theory for representative set. The hindrance potential and HO/HR values for selected processes. Relative absolute deviations as functions of the temperature between rate constants obtained with explicit RC-TST formulation. (PDF 3754 kb)
Rights and permissions
About this article
Cite this article
Baradyn, M., Ratkiewicz, A. Kinetics of the hydrogen abstraction alkane + O2 → alkyl + HO2 reaction class: an application of the reaction class transition state theory. Struct Chem 31, 731–746 (2020). https://doi.org/10.1007/s11224-019-01459-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-019-01459-x