Abstract
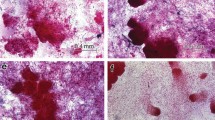
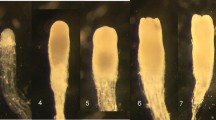
Somatic embryogenesis (SE) is expected to play an important role in the future of US forests by providing increased productivity, sustainability, and uniformity. For broad scale implementation to occur, SE technology must work with a variety of genetically diverse trees. Douglas fir (Pseudotsuga menziesii (Mirb) Franco) is the dominant tree in the Pacific Northwest and has great economic and recreational value. We have developed a highly effective medium for initiation of embryogenic tissue of Douglas fir that contains ABA, biotin, brassinolide, folic acid, MES, pyruvic acid and can be used as a gelled medium or in a gelled-liquid medium overlay system. When tested with many high-value crosses over 2 years, initiation tests averaged initiation in the range of 40–57%. Additionally, a time- and labor-saving tetrazolium chloride embryo staining technique was developed to evaluate seed health and screen out seed sources likely to perform poorly in the initiation process.



Similar content being viewed by others
Abbreviations
- ABA:
-
Abscisic acid
- AC:
-
Activated carbon
- BAP:
-
6-Benzylaminopurine
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- DF:
-
Douglas fir
- MES:
-
2(N-morpholino)ethanesulfonic acid buffer agent
- SE:
-
Somatic embryogenesis
References
Aitken-Christie J, Parkes BD (1996) Improved embryogenesis process for initiation and maturation. International application under the patent cooperation treaty (PCT). PCT Pub No. WO 97/37046, PCT publication date: 28 November 1996
Becwar MR, Pullman GS (1995) Somatic embryogenesis in loblolly pine (Pinus taeda L.). In: Mohan Jain S, Gupta PK, Newton RJ (eds) Somatic embryogenesis in woody plants, Vol. 3—Gymnosperms. Kluwer, The Netherlands, pp 287–301
Cairney J, Pullman GS (2007) The cellular and molecular biology of conifer embryogenesis. New Phytol 176:511–536. doi:10.1111/j.1469-8137.2007.02239.x
Cairney J, Xu N, MacKay J, Pullman G (2000) Transcript profiling: a tool to assess the development of conifer embryos. In vitro Cell Dev Biol Plant 36:155–162. doi:10.1007/s11627-000-0031-5
Carman JG, Reese G, Fuller RJ, Ghermay T, Timmis R (2005) Nutrient and hormone levels in Douglas-fir corrosion cavities, megagametophytes, and embryos during embryony. Can J For Res 35:2447–2456. doi:10.1139/x05-173
Chalupa V (1985) Somatic embryogenesis and plantlet regeneration from cultured immature and mature embryos of Picea abies (L.) Karst. Commun Inst For Chech 14:57–63
Chiwocha S, von Aderkas P (2002) Endogenous levels of free and conjugated forms of auxin, cytokinins and abscisic acid during seed development in Douglas fir. Plant Growth Regul 36:191–200. doi:10.1023/A:1016522422983
Durzan DJ, Gupta PK (1987) Somatic embryogenesis and polyembryogenesis in Douglas-fir cell suspension cultures. Plant Sci 52:229–235. doi:10.1016/0168-9452(87)90056-2
Grano CX (1958) Tetrazolium chloride to test loblolly pine seed viability. For Sci 4:50–53
Gupta PK, Grob JA (1995) Somatic embryogenesis in conifers. In: Jain S, Gupta P, Newton R (eds) Somatic embryogenesis in woody plants, vol 1. Kluwer Academic, Dordrecht, The Netherlands, pp 81–98
Gupta PK, Holmstrom DG, Budworth D (2004) Embryogenic culture initiation of Douglas fir by maltose. US Patent Application No. 20040237130
Hackman I, von Arnold S (1985) Plantlet regeneration through somatic embryogenesis in Picea abies (Norway spruce). J Plant Physiol 121:149–158
Handley LW (1997) Method for regeneration of coniferous plants by somatic embryogenesis in culture media containing abscisic acid. US Patent 5,677,185
Handley L III (1999) Method for regeneration of coniferous plants by somatic embryogenesis in culture media containing abscisic acid. US Patent 5,856,191, 5 Jan 1999
Hong L, Boulay M, Gupta PK, Durzan DJ (1991) Variations in somatic polyembryogenesis: induction of adventitious embryonal-suspensor masses on developing Douglas fir embryos. In: Ahuja MR (ed) Woody plant biotechnology. Plenum, New York, USA, pp 105–121
Kapik RH, Dinus RJ, Dean JF (1995) Abscisic acid and zygotic embryogenesis in Pinus taeda. Tree Physiol 15:405–409
Kong L, Attree SM, Evans DE, Binarova P, Yeung EC, Fowke LC (1999) Somatic embryogenesis in white spruce: studies of embryo development and cell biology. In: Mohan Jain S, Gupta PK, Newton RJ (eds) Somatic embryogenesis in woody plants, vol 4. Kluwer, The Netherlands, pp 1–28
Malabadi R, Nataraja K (2007) 24-Epibrassinolide induces somatic embryogenesis in Pinus wallichiana A. B. Jacks. J Plant Sci 2:171–178
Mattson JS, Mark SB (1971) Activated carbon surface chemistry and adsorption from solution. Marcel Dekkar, New York
Nagmani R, Bonga JM (1985) Embryogenesis in subcultured callus of Larix decidua. Can J Res 15:1088–1091. doi:10.1139/x85-177
Pullman GS, Gupta PK (1994) Method for reproducing conifers by somatic embryogenesis using mixed growth hormones for embryo culture. US Patent 5,294,549, issued 15 Mar 1994
Pullman GS, Johnson S (2002) Somatic embryogenesis in loblolly pine (Pinus taeda L.): improving culture initiation rates. Ann Sci 59:663–668. doi:10.1051/forest:2002053
Pullman GS, Peter G (2002) Methods of initiating embryogenic cultures in plants. US Patent 6,492,174B1
Pullman GS, Peter G (2006) Methods for increasing conifer somatic embryo initiation, capture, and multiplication. US Patent Application No. 20060051868, 9 Mar 2006
Pullman GS, Skryabina A (2007) Liquid medium and liquid overlays improve embryogenic tissue initiation in conifers. Plant Cell Rep 26:873–887. doi:10.1007/s00299-006-0296-1
Pullman GS, Webb DT (1994) An embryo staging system for comparison of zygotic and somatic embryo development. TAPPI R&D Division Biological Sciences Symposium, Minneapolis, Minnesota, 3–6 October 1994, pp 31–34
Pullman GS, Cairney J, Peter G (1998) Clonal forestry and genetic engineering: where we stand, future prospects, and potential impacts on mill operations. TAPPI J 81:57–64
Pullman GS, Johnson S, Peter G, Cairney J, Xu N (2003a) Improving loblolly pine somatic embryo maturation: comparison of somatic and zygotic embryo morphology, germination, and gene expression. Plant Cell Rep 21:747–758
Pullman GS, Namjoshi K, Zhang Y (2003b) Somatic embryogenesis in loblolly pine (Pinus taeda L.): improving culture initiation with abscisic acid, silver nitrate, and cytokinin adjustments. Plant Cell Rep 22:85–95. doi:10.1007/s00299-003-0673-y
Pullman GS, Zhang Y, Phan B (2003c) Brassinolide improves embryogenic tissue initiation in conifers and rice. Plant Cell Rep 22:96–104. doi:10.1007/s00299-003-0674-x
Pullman GS, Gupta PK, Timmis R, Carpenter C, Kreitinger M, Welty E (2005a) Improved Norway spruce somatic embryo development through the use of abscisic acid combined with activated carbon. Plant Cell Rep 24:271–279. doi:10.1007/s00299-005-0933-0
Pullman GS, Johnson S, Van Tassel S, Zhang Y (2005b) Somatic embryogenesis in loblolly pine (Pinus taeda L.) and Douglas fir (Pseudotsuga menziesii): Improving culture initiation with MES pH buffer, biotin, and folic acid. Plant Cell Tissue Organ Cult 80:91–103. doi:10.1007/s11240-004-9099-7
Pullman GS, Mein J, Johnson S, Zhang Y (2005c) Gibberellin inhibitors improve embryogenic tissue initiation in conifers. Plant Cell Rep 23:596–605. doi:10.1007/s00299-004-0880-1
Pullman GS, Chopra R, Chase K-M (2006) Loblolly pine (Pinus taeda L.) somatic embryogenesis: improvements in embryogenic tissue initiation by supplementation of medium with organic acids, vitamins B12 and E. Plant Sci 170:648–658. doi:10.1016/j.plantsci.2005.10.018
Pullman GS, Chase K-M, Skryabina A, Bucalo K (2008) Conifer embryogenic tissue initiation: improvements by supplementation of medium with d-chiro-inositol and d-xylose. Tree Physiol, in press (accepted 9/10/2008)
USDA Forest Inventory and Analysis National Program (2008) http://www.fia.fs.fed.us/. Accessed 30 June 2008
Van Winkle SC, Pullman GS (2003) The combined impact of pH and activated carbon on the elemental composition of plant tissue culture media. Plant Cell Rep 22:303–311. doi:10.1007/s00299-003-0686-6
Western Wood Products Association (2008) http://www2.wwpa.org/WESTERNSPECIES/DouglasFir/tabid/405/Default.aspx. Accessed 30 June 2008
Xu N, Johns B, Pullman GS, Cairney J (1997) Rapid and reliable differential display from minute amounts of tissue: mass cloning and characterization of differentially expressed genes from loblolly pine embryos. Plant Mol Biol Rep 15:377–391. doi:10.1023/A:1007437911234
Acknowledgments
We thank the member companies of the Institute of Paper Science and Technology (IPST) and IPST at Georgia Tech for financial support and The Timber Company and Weyerhaeuser Company for cones. We are grateful for the help of J. Dregar, S. Duncan, J. Grabowski, R. Gupta, J. Halpin, R. Howie, C. Umejiego, and Y. Zhang.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pullman, G.S., Johnson, S. & Bucalo, K. Douglas fir embryogenic tissue initiation. Plant Cell Tiss Organ Cult 96, 75–84 (2009). https://doi.org/10.1007/s11240-008-9462-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-008-9462-1