Abstract
To test the possibility of producing a novel hepatitis B vaccine in plants, the modified hepatitis B virus (HBV) surface antigen (HBsAg) gene SS1 was expressed in rice under the control of the seed-specific Glub-4 promoter. The SS1 gene encodes a fusion protein consisting of amino acids 21–47 of the hepatocyte receptor-binding presurface 1 region (preS1) fused to the truncated C-terminus of the major HBV surface (S) protein. The production of antibodies against the preS1 region acts to protect humans against HBV infection by preventing HBV from binding to hepatocytes. The presence of SS1 in the genome of transgenic rice was confirmed by PCR and Southern blot analysis, and RNA dot blot analysis indicated that the fused SS1 gene was specifically expressed in rice seeds, with the highest expression level being about 31.5 ng/g dry weight grain. Western blot analysis revealed that the recombinant SS1 protein could be specifically recognized by both an anti-S protein antibody and an anti-preS1 antibody. The recombinant SS1 protein was also observed to form virus-like particles with a diameter of about 22 nm and a density of 1.25 g cm−3. Furthermore, immunological responses against both the S and preS1 epitopes were induced in BALB/c mice immunized with the recombinant SS1 protein, indicating that this rice-derived SS1 protein could be a promising candidate as an alternative HBV vaccine for preventing hepatitis B.
Similar content being viewed by others
Introduction
Hepatitis B (HB) ranks among the severest chronic diseases in the world, especially in tropical Africa and Far-East Asia. Currently, about 300 million people worldwide carry hepatitis B virus (HBV), which can lead to chronic hepatic cirrhosis and hepatocellular carcinoma (Milich 1997). Since the introduction of a commercial recombinant vaccine derived from yeast, the spread of HBV infection has been greatly slowed. However, the expense of current immunization programs limits their use for large populations in developing countries. Also, around 10% of adults have no response to current commercial vaccines, and 5–10% show only a low response (Valenzuela et al. 1982). Thus, research has focused on the production of recombinant HBV surface antigen (rHBsAg) in plants with the hope of developing a less expensive HBV vaccine for oral delivery. Mason and co-workers have demonstrated convincingly that plant-derived rHBsAg can self-assemble into a virus-like particle (VLP) with mucosal immunogenicity in both mice and humans (Mason et al. 1992; Thanavala et al. 1995, 2005; Dogan et al. 2000; Richter et al. 2000; Kong et al. 2001). However, their plant-derived rHBsAg subunit vaccine is composed mainly of the small protein (S) of HBV, which is similar to the commercial vaccine derived from yeast (Mason et al. 1992).
The lack of, or low, response to S protein vaccination occurs not only in cases of obesity, renal failure or immune suppression, but also in healthy individuals (Iwarson 1995). Accordingly, it is necessary to develop a more effective vaccine against HBV. It has been shown that it is the presurface (preS) region of HBsAg, rather than S protein, that plays an important role in the interaction between HBV and hepatocytes. A receptor binding site for hepatocytes was found within amino acids (aa) 21–47 of the preS1 sequence (Neurath et al. 1986). Anti-serum raised against a synthetic preS1 peptide (aa21–47) was able to neutralize the virus by blocking virus attachment to cells, and antibodies to a synthetic peptide mimicking preS1 (aa21–47) could protect chimpanzees from HBV infection (Neurath et al. 1989). Therefore, inclusion of the preS1 antigen in the vaccine may enhance its protective efficacy. We previously described the synthesis and assembly of a modified HBsAg carrying preS1 (SS1), in which preS1 (aa21–47) was fused to the truncated C-terminus of the S protein at amino acid position 223, in vaccinia virus and yeast expression systems (Xu et al. 1994; Hui et al. 1999; Yang et al. 2000a, b). The data obtained suggested that the recombinant SS1 protein could be a promising candidate for a novel recombinant vaccine, inducing the broader immunological response required for protection against HBV infection.
Rice is an important staple crop in China and other Asian countries. It is considered hypoallergenic, and rice-based infant formulas are commercially available. Also, as rice is a self-pollinating crop, its pollen viability and out-crossing rates are very low, reducing the segregation requirement and the chance of gene flow via pollen when working with transgenic plants (Huang and Yang 2005). Thus, rice is particularly suitable for the production of recombinant proteins for oral applications. In this paper, we demonstrate that recombinant SS1 protein produced in rice seeds can form VLP structures with both S and preS1 immunogenicity, indicating that this recombinant SS1 protein might have potential as a novel, edible, HBV candidate vaccine.
Materials and methods
Materials
Rice seeds (Oryza sativa L. cv. Zhonghua No.11, kindly provided by the Shanghai Academy of Agricultural Sciences, China) were used for transformation. The DNA oligonucleotides listed in Table 1 were synthesized by Invitrogen (Shanghai, China).
Construction of a rice endosperm-specific vector expressing SS1
In order to produce recombinant SS1 protein in rice seeds, the endosperm-specific 1.4 kb GluB-4 promoter (GenBank gi:42742293) together with its 5′ UTR (untranslated region) was isolated from rice genomic DNA by PCR using the specific primers PGluB4F and PGluB4R (Table 1). The coding sequence of the GluB-1 signal peptide (SP; GenBank gi:20231) was also obtained by fusing two complementary oligonucleotides (GluB1SPF and GluB1SPR) to the 3′ end of the GluB-4 promoter using overlapping PCR. The resulting fragment was cloned into the vector pMD18-T (Takara, Japan) and confirmed by sequencing. A sequence encoding the endoplasmic reticulum retention signal ‘KDEL’ was added to the SS1 gene at the 3′ end using primers SS1F and SS1R based on the SS1 nucleotide sequence information in the plasmid pCDNASS1 (Yang et al. 2000b). The 660 bp 3′ UTR of GluB-1, which was amplified from rice genomic DNA by PCR using specific primers GluB13UTRF and GluB13UTRR (GenBank gi:58530788), was used as a terminator. The two resulting PCR fragments were also cloned into pMD18-T and confirmed by sequencing. The 660 bp 3′ UTR of the GluB-1 fragment in pMD18-T was cleaved by EcoRI and XhoI and ligated into pCAMBIA1300 (Clontech, Palo Alto, CA) to generate pUTR. The modified SS1 gene (780 bp in length) was inserted between the EcoRI and BamHI sites of pUTR, generating pSS1UTR. The Xho I–BamHI fragment containing the GluB-4 promoter with its 5′ UTR and the SP coding sequence of the GluB-1 gene was then cloned into the SalI and BamHI sites upstream of the modified SS1 gene in pSS1UTR to generate pPSS1UTR. Finally, the 2.8 kb SS1 expression cassette released by digesting pPSS1UTR with XhoI and HindIII was cloned into pCAMBIA1300 to generate the plant binary vector p1300GSS1 (see Supplemental Figure 1). All enzymatic digestions, ligations, and other DNA manipulations were performed according to standard protocols (Sambrook et al. 1989).
Production of transgenic plants
Transgenic rice plants (Oryza sativa cv. Zhonghua No.11) were produced by Agrobacterium-mediated transformation, following the protocol described by Liu et al. (1998). Briefly, plasmid p1300GSS1 was introduced into Agrobacterium tumefaciens strain EHA105 by electroporation. Two-week-old calli derived from mature rice seeds were treated with the transformed A. tumefaciens for 3 days. The infected calli were successively cultured for 4 weeks each in N6 selection and MS regeneration medium containing 500 mg/l cefotaxime and 25 mg/l hygromycin. The regenerated seedlings were transplanted to the field.
DNA analysis
The presence of the target gene in transgenic plants was confirmed by PCR analysis and Southern blot analysis. Samples of genomic DNA (~20 ng), extracted from the leaves of rice plants by the CTAB method (Doyle and Doyle 1987), were used as templates in PCR reactions using the SS1 gene-specific primers SS1F and SS1R, with the expected PCR products being 780 bp in length. Genomic DNA (10 μg) was digested with SalI to further confirm the presence of the inserted T-DNA for p1300GSS1-derived transformants. Digested DNA was separated by electrophoresis on 1% agarose gels, transferred to nylon membrane (Hybond N+, Amersham Biosciences, Piscataway, NJ), and hybridized with a random-primed 32P-labeled 780 bp PCR-amplified SS1 DNA fragment from plasmid p1300GSS1 (labeled using the Prime-a-Gene Labeling System; Promega, Madison, WI).
To confirm the results of Southern blot analysis, real-time PCR using SYBGreen I dye was performed. The SPS gene encoding the sucrose phosphate synthase (SPS) in rice was used as an endogenous reference gene to determine the copy numbers of the exogenous SS1 genes in transgenic plants (Ding et al. 2004; Yang et al. 2005). The SPSF/SPSR primer pair for SPS and the QHBVF/QHBVR primer pair for SS1 (Table 1) were employed for quantitative PCR analysis, yielding amplified fragments of 81 and 90 bp, respectively. Each reaction had three replicates and was repeated three times. Transgene copy number was calculated according to the method described by Weng et al. (2004).
RNA dot blot analysis
To test whether the SS1 gene was transcribed in the transgenic plants, RNA dot blot analysis was performed. Total RNA was isolated from maturing seeds 10 DAF (days after flowering), leaves, or roots using a Trizol Kit (Promega) following the manufacturer’s recommendations (Tada et al. 2003). Briefly, 5 μg total RNA in 10 μl was mixed with 6 μl 20 × SSC and 4 μl formaldehyde, heated to 68°C for 10 min and quickly cooled on ice for 5 min; the samples were then dotted onto a prewetted nylon membrane (Hybond N+, Amersham Biosciences) (Wei et al. 1995). The resulting nylon membrane was then hybridized with the random-primed 32P-labeled SS1 gene probe described above.
Detection and quantification of recombinant SS1 protein in rice seeds
To determine expression levels, the amount of recombinant SS1 protein in transgenic rice seeds was measured by quantitative ELISA (Dutauda et al. 2002). Transgenic rice seeds were milled (DADE, China) into a fine powder; the powder was soaked with 1.5 times the volume of seed weight (ml/g) of ice-cold buffer containing phosphate-buffered saline (PBS, pH 7.4), 10 mM EDTA, 0.1% Triton X-100, and 1 mM phenylmethylsulfonyl fluoride at 4°C overnight, then sonicated on ice and centrifuged at 13,000 rpm. The concentration of total soluble protein (TSP) in the supernatant was determined by Bradford's method with bovine serum albumin as a standard (Bradford 1976). Recombinant SS1 protein in TSP was quantified using an HBsAg detection kit (Shanghai Kehua Bioengineering, Shanghai, China) with the standard for S protein (National Institute for the Control of Pharmaceutical and Biological Products, China) according to the manufacturer’s recommendations, with a correction coefficient of 1.21 (the molecular weight ratio of SS1 to S). To further confirm the accumulation of SS1 protein in transgenic rice seeds, 100 μg total seed protein was separated on 15% SDS-PAGE, and then transferred to Hybond-P poly (vinylidene difluoride) membranes (Schleicher & Schuell Bioscience, Dassel, Germany) for Western blot analysis according to the manufacturer’s recommendations. The S protein (kindly provided by Shanghai Kehua Bioengineering) and the purified fusion preS1 protein were used as positive controls (Qian et al. 2006). The membranes were probed with mouse anti-S peptide monoclonal antibody (McAb) (Biodesign, Kennebunk, ME) and preS1 (21–47aa) epitope monoclonal antibody (kindly provided by Prof. Zhuchuan Zhang, Institute of Biochemistry and Cell biology, Shanghai Institute of Biological Sciences, the Chinese Academy of Sciences, Shanghai, China) as the primary antibodies (1:2,000), respectively. The membrane was then incubated with a goat anti-mouse IgG (H + L) secondary antibody conjugated to alkaline phosphatase (Promega) (1:5,000) to visualize signals (Huang et al. 2003).
CsCl gradient centrifugation analysis of recombinant SS1 protein
To assess whether the recombinant SS1 in rice forms VLP structures, recombinant SS1 protein in TSP prepared as described above was partially purified through a Sepharose-4B column (Gao et al. 1998). About 5 ml of the collected samples containing recombinant SS1 protein were diluted to 10 ml by adding 10 g CsCl dissolved in 5 ml PBS (pH 7.4) and mixed gently (Yang et al. 2000b). Centrifugation was performed at 45,000 rpm for 48 h (Beckman, SW41i Rotor) at 5°C. Fractions of 400 μl collected from the gradient were assayed for S and preS1 antigens by indirect ELISA (Yang et al. 2000b). The density of the gradient fractions was calculated by measuring the refractive index using an Abbe refractometer. Samples collected from the peak fractions were examined by electron microscopy.
Solid phase immune electron microscopy analysis
To directly observe VLPs in the recombinant SS1 fraction, samples were analyzed by solid phase immune electron microscopy (SPIEM). Carbon copper-coated grids were floated on a drop of pooled anti-S McAb (20 μg/ml in PBS, pH 7.4) for 10 min at 4°C in a moist chamber, followed by washing with six sequential drops of PBS. The grids were then floated on the protein samples for 30 min at 37°C in the same moist chamber. Finally, the grids were washed with six drops of PBS, then negatively stained with phosphotungstic acid and viewed by electron microscopy following a standard protocol (Lewis 1990).
Immunization of BALB/c mice
The immunogenicity of the recombinant SS1 protein was tested in BALB/c mice. Mice were purchased from the animal facility of Shanghai Medical College of Fudan University (Shanghai, China). A group of eight adult BALB/c mice (female) aged 6–8 weeks were immunized intraperitoneally three times at 2-week intervals with freeze-dried total protein of all transgenic rice seeds. Each dose—about 0.5 μg recombinant SS1 protein—was emulsified with 200 μl complete Freund’s adjuvant (CFA) in final volume of 400 μl at the first immunization, and with incomplete Freund’s adjuvant (IFA) at subsequent immunizations (Thanavala et al. 1995). Another group of eight adult BALB/c mice were immunized with the total protein of non-transgenic rice seeds as negative controls with the same immunizing schedule. Blood collected from mice before immunization, and at weekly intervals thereafter over a period of 12 weeks, was analyzed for the presence of antibodies against S and preS1.
Analysis of anti-S and anti-preS1 antibodies in mice sera
Since one of the ultimate aims of this study is to generate recombinant protein with S and preS1 epitope immunogenicity, specific serum antibodies against the S and preS1 epitope were examined by indirect ELISA as described by Yang et al. (2000a). The national standard for S and the purified fusion His6preS1 protein derived from bacteria were used as the coating antigens for the 96-well plates for S and preS1, respectively. Mouse serum IgG was tested in a 10-fold dilution series in the blocking buffer. Alkaline phosphatase conjugated to goat anti-mouse IgG (H + L) (Promega) was used as secondary antibody (diluted 1:10,000 in blocking buffer). After incubation with 75 μl NPP-Na salt solution for 30 min at room temperature to develop color, the reaction was stopped by adding 25 μl 0.5 M NaOH, and the optical density (OD) was measured at dual wavelengths of 450:630 nm (Huang et al. 2005a). The mean value of antibody titers was determined by the serial end-point dilution method. End-point titers were defined as OD 450:630 nm at the highest serum dilution for sera from the group of mice immunized with the rice seed-derived recombinant SS1 protein. The absorbance values at this dilution should satisfy the requirement of being 2.1 times greater than that of serum from control mice immunized with total protein of non-transgenic rice seeds (Yang et al. 2000b).
Results
Construction of the expression vector p1300GSS1 and transformation of rice plants
Although SS1 has been successfully expressed in mammalian cells and yeast, and has been shown to be immunogenic (Xu et al. 1994; Hui et al. 1999; Yang et al. 2000a, b), little is known regarding its expression and immunogenicity in plants. To test the possibility of production of SS1 in plants as a novel HBV vaccine candidate, we produced SS1 in rice seeds. The binary vector p1300GSS1 was constructed based on the pCAMBIA13000 vector used for rice transformation (see Supplemental Figure 1). The SS1 gene was under the control of the GluB-4 promoter, thus accumulation of SS1 protein in rice seeds was expected. To target recombinant SS1 protein to the protein storage particles, the vector incorporated the 5′ UTR and signal peptide of the GluB-1 gene. A sequence encoding the endoplasmic reticulum retention signal ‘KDEL’ was fused to the C terminus of SS1 to enhance SS1 accumulation levels (Hirahara et al. 2001). The modified SS1 gene was terminated by the 3′ UTR of GluB-1, and the resultant plasmid p1300GSS1 was transferred into A. tumefaciens EHA105 for plant transformation.
Thereafter, transgenic rice plants were obtained via Agrobacterium-mediated transformation. Among the 416 regenerated plants obtained, 164 were transgenic for the SS1 gene as shown by PCR analysis (data not shown). When transgenic plants reached a height of 10 cm, they were transferred to field conditions, where they exhibited normal growth compared to non-transgenic rice plants.
Southern blot and RNA dot blot analysis of transgenic rice
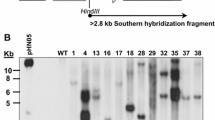
Among 164 transgenic plants, 1 (transgenic plant #25) exhibited a higher expression level of recombinant SS1 protein (see below). The DNA copy number of the integrated DNA in this transgenic plant was determined by Southern blot analysis. Genomic DNA from transgenic plant #25 was digested with SalI, transferred to nylon membrane, and hybridized with a random-primed 32P-labeled SS1 gene probe. Two hybridization bands of about 5 and 8 kb were detected (Fig. 1A). There is a single SalI restriction enzyme site within the T-DNA region of the expression vector p1300GSS1, so we propose that at least two copies of SS1 were inserted in this transgenic plant genome. This result was further confirmed by real-time PCR, which confirmed that two copies of the SS1 gene have been inserted in the genome of transgenic plant #25 (see Supplemental Figure 2 and Supplemental Table 1).
DNA and RNA analysis of transgenic plant #25. (A) Southern blot analysis. Genomic DNA isolated from fresh leaves of transgenic plant #25 was digested with SalI, fractionated by electrophoresis on a 1% agarose gel, transferred to nylon membrane and hybridized with a random-primed 32P-labeled SS1 gene probe. Lanes: 1 Non-transgenic rice, 2 transgenic plant #25. (B) RNA dot hybrid analysis of SS1 gene in transgenic plant #25. Total RNA was isolated from fresh leaves (L), roots (R) and developing seeds (S). Rows: 1 Transgenic plant #25; 2, wild type
To investigate whether the SS1 gene was expressed specifically in rice seeds, total RNA derived from maturing seeds (10 DAF), leaves, and roots of transgenic plant #25 and a non-transgenic plant were used in RNA dot blot analysis. SS1 transcripts were detected only in the maturing seeds of transgenic plant #25, whereas no signal was observed in the leaves or roots of this transgenic plant or in non-transgenic plants (Fig. 1B). This result indicated that the SS1 gene is specifically expressed in seeds under the control of the Glub-4 promoter.
Expression level of recombinant SS1 protein in rice seeds
To determine the accumulation levels of recombinant SS1 protein expressed in transgenic rice seeds, quantitative ELISA analysis was performed to measure the recombinant antigen in TSP from seeds of all transgenic rice plants. No HBsAg was detected in the sample derived from wild-type rice seeds, so this sample was used as a control. A standard curve was established using different dilutions of standard S protein, and a linear dose–response curve was obtained over the range 1–5 ng/ml, with a coefficient of determination r 2 of 0.9994 and a slope of 1.522 (data not shown). Of the 164 plants tested, transgenic plant #25 accumulated the highest level of recombinant SS1 protein at ~31.5 ± 1.4 ng (mean ± standard error of the mean) per gram dry weight (DW) grain. Several plants showed slightly lower expression levels, ranging from 15.8 ± 0.7 to 26.3 ± 1.1 ng/g DW grain. In addition, five transgenic plants showed no SS1 accumulation, likely due to silencing of the SS1 gene in the transgenic plants.
Antigenicity of the recombinant SS1 protein
To determine the antigenicity of the recombinant SS1 protein, Western blot analysis was performed using antibodies against S and preS1 protein. As shown in Fig. 2, both antibodies specifically recognised a protein with a molecular mass of 29 kDa, which is consistent with the estimated molecular weight of SS1 protein, in transgenic plant #25 (Fig. 4, lanes 3). No signal cross-reacting with anti-S or anti-preS1 antibodies was observed in a wild-type plant (Fig. 4, lanes 2). The fact that the modified protein could be recognized by both anti-S and anti-preS1 antibodies indicates that this rice-derived recombinant SS1 protein possesses both S and preS1 antigenicity.
Western blot analysis of recombinant SS1 protein. (A) Western blot using preS1 monoclonal antibodies (McAb) for detection. Lanes: M Protein markers, 1 1 μg purified fusion protein His6preS1 expressed in Escherichia coli, 2 100 μg total protein from non-transgenic rice, 3 100 μg total protein from transgenic plant #25. (B) Western blot using S McAb for detection. Lanes: M Protein markers, 1 10 ng S protein (provided by Shanghai Kehua Bioengineering), 2 100 μg total protein from non-transgenic rice, 3 100 μg total protein from transgenic plant #25
Analysis of VLPs formed by rice-derived recombinant SS1 protein
VLPs are produced upon recombinant expression of viral capsid or envelope proteins, which assemble into highly immunogenic virion-like structures devoid of viral genetic material. Formation of VLPs has been shown to be critical for inducing a strong immunological response. rHBsAg protein expressed in yeast and plants has been shown to induce an immunological response (Mason et al. 1992). Moreover, recombinant SS1 protein expressed in yeast can self-assemble into VLPs (Yang et al. 2000b). To examine whether recombinant SS1 protein formed VLPs in this study, partially purified SS1 protein from rice seeds was subjected to CsCl density gradient centrifugation, and gradient fractions were assayed for S and preS1 antigenicity by ELISA. Signals of S and preS1 were both observed at a density of about 1.25 g cm−3 (Fig. 3A), which was slightly higher than the 1.20 g cm−3 of naked spherical S protein particles (Tiollais et al. 1985) and the 1.23 g cm−3 of SS1 expressed in yeast (Yang et al. 2000a). Samples with positive S and preS1 signals were observed further under transmission electron microscopy (TEM). Spherical particles with a diameter of about 22 ± 2 nm could be observed (Fig. 3B), similar to S particles expressed in tobacco and SS1 previously expressed in yeast (Mason et al. 1995; Yang et al. 2000a). These results show that the recombinant SS1 protein could self-assemble into VLPs.
Analysis of virus-like particle (VLP) structure of recombinant SS1 protein. (A) CsCl gradient centrifugation analysis of purified SS1 protein. Open circles Density of CsCl, filled diamonds antigenicity of preS1, open squares antigenicity of S. (B) Solid phase immune electron microscopy (SPIEM) analysis of the upper peak fraction; particles were visualized after negative staining
Immunogenicity of recombinant SS1 protein in BALB/c mice
Immunological response to the recombinant SS1 protein was assayed in BALB/c mice. As shown in Fig. 4, recombinant SS1 from transgenic rice could elicit a specific antibody response against both S and preS1 in mice immunized intraperitoneally at days 0, 14, 28 with crude protein extracted from rice seeds containing about 0.5 μg recombinant SS1 protein. Specific preS1 antibodies were first detected in immunized mice 4 weeks after the initial antigen injection. The response rose steadily, reaching a peak value of 0.475 ± 0.055 (OD450:630) 3 weeks after the final boosting injection. Specific S antibodies were first detectable 5 weeks after the initial antigen injection; the level of antibodies increased significantly and reached a maximum value of 0.446 ± 0.053 (OD450:630) in the 8th week. Antibodies against both S and preS1 remained at relatively high levels during subsequent weeks. Mice immunized with crude protein from non-transgenic rice seeds showed no immune response to S and preS1. The immunological response to rice-derived SS1 seems lower than that produced by yeast-derived recombinant SS1 (Yang et al. 2000a).
Immunological response elicited by immunization of BALB/c mice with recombinant SS1 protein derived from rice seeds. Results are expressed as OD 450:630 nm of a 1:100 dilution of sera from mice immunized with rice seed-derived recombinant SS1 protein. This dilution is the highest serum dilution at which absorbance values still satisfy the requirement of being 2.1 times greater than that of control serum from mice immunized with total protein of non-transgenic rice seeds. Data are the means of values obtained for each group of immunized mice. Bars Standard error, arrows times of immunization
Discussion
In recent years, the use of plants as bioreactors has become of special interest, as plants allow production of recombinant proteins in large quantities at relatively low cost (Mason et al. 1995, 2002). Since the 1990s, researchers have attempted the expression of rHBsAg in tobacco, potato, cherry, tomato, and lettuce (Streatfield 2005), with the hope of developing an oral vaccine to prevent HBV infection. Indeed, rHBsAg has been successfully expressed in leaf and tuber expression systems and in plant cell culture (Streatfield 2005). Among these studies, the most successful example expressed rHBsAg in potato, which has now been used in clinical I trials (Thanavala et al. 2005).
In this study, SS1 was expressed under the control of the seed-specific GluB-4 promoter, and an endoplasmic reticulum retention signal (KDEL) was also used to enhance accumulation of SS1. Compared with previous studies of expression of recombinant rHBsAg in plants, the expected increased expression level of SS1 was not observed, with levels of about 0.000003% of grain weight being obtained. In transgenic tobacco, the HB major surface antigen was first expressed in tobacco leaves at less than 0.01% of TSP (Mason et al. 1992), and in lettuce leaves, its expression level is less than 0.000001% of fresh weight (Kapusta et al. 1999). In the potato tuber expression system, an expression level of about 0.002% of fresh weight was obtained following a variety of optimization strategies (Richter et al. 2000). We propose that the low expression level of SS1 might be due to poor codon usage of the SS1 gene in rice (Huang and Yang 2005).
Interestingly, despite its low expression level in rice, we proved that the recombinant SS1 protein derived from rice seeds had both S and preS1 antigenicity simultaneously, and could self-assemble into VLPs. Such SS1 VLPs are thought to be suitable for oral immunization. With oral delivery of a vaccine, stability in the gut with its many kinds of digestive enzymes is very important. In addition, the ability to be efficiently transported from the gut lumen into the gut-associated lymphoid tissue (GALT) for antigen processing and presentation to stimulate protective immune response is also critical. Analysis of Norwalk VLPs produced in either a cell-free translation system or in recombinant baculovirus showed that the derived VLPs were protease resistant (Jiang et al. 1992), and that VLP structure might protect the antigen from being degraded by digestive enzymes in the gut. Moreover, some researchers have proposed that the particulate nature of VLPs would allow them to be efficiently sampled by the “M” cells of the gut epithelium overlying the GALT, and to be transported across the mucosal barrier (Huang et al. 2005b). Importantly, these VLP structures, which mimic the authentic viral particle, might act as a “danger signal” that could overcome being taken as benign by the immune system, and thus effectively provoke an immune response (Huang et al. 2005b). Thus, the VLPs formed by recombinant SS1 protein produced in rice could represent a good candidate vaccine for further study.
Moreover, immunological responses against both the S protein and preS1 epitope were induced in BALB/c mice immunized with recombinant SS1 protein. The response to rice-derived SS1 was slightly lower than the immunological response elicited by highly purified SS1 expressed in yeast (Yang et al. 2000b). The reason for this might be due to the crude protein used for immunization. This phenomenon was also observed in the S protein from tobacco (Thanavals et al. 1995). In our previous study, we did not observe any augmentation of anti-S antibody by the inclusion of preS1 epitopes. However, a strong and rapid immune response to preS1 could be elicited by recombinant SS1 protein (Hui et al. 1999). The concept of an attachment blockage pathway of virus neutralization suggests that an effective immune response to the virus surface protein sequence involved in target cell recognition is an important component of the virus-neutralizing mechanism of the host. Hence it is proposed that the preS1(aa21–47) region involved in such receptor recognition is significant in immunization (Neurath et al. 1986). Our results suggest that modified particles containing preS1 epitopes, which elicited an anti-S response as well as an anti-preS1 response, could provide a promising candidate for a novel HBV vaccine that would induce the broader range of antibodies required for protection against HBV infection.
LT-B expressed in corn seed in the form of a pentamer is much more resistant to heat than the pure protein (Streatfield et al. 2002). Furthermore, anti-S antibodies could also be elicited by oral immunization with boiled potato containing rHBsAg (Thanavala et al. 1995). These exciting reports encourage us to enhance the expression level of our recombinant SS1 protein in rice seeds and to pursue further immunological analysis, for instance, the oral immunogenicity of this SS1 protein, to achieve our aim of producing a novel recombinant vaccine to induce the broad immunological response required for protection against HBV infection.
In conclusion, in this study we expressed recombinant SS1 protein in rice seeds. The expressed SS1 could self-assemble into VLPs with both S and preS1 antigenicity. Moreover, the recombinant SS1 protein could induce immunological responses against S and preS1 protein in mice, implying that this rice-derived SS1 protein could be developed as an alternative oral vaccine for preventing HBV infection in humans.
References
Bradford MM (1976) A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Ding JY, Jia JW, Yang LT, Weng HB, Zhang CM, Liu WX, Zhang DB (2004) Validation of a rice specific gene, sucrose phosphate synthase, used as the endogenous reference gene for qualitative and real-time quantitative PCR detection of transgenes. J Agric Food Chem 52:3372–3377
Dogan B, Mason HS, Richter L, Hunter JB, Shuler ML (2000) Process options in hepatitis B surface antigen extraction from transgenic potato. Biotechnol Prog 16:435–441
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Dutauda D, Aubrya L, Henryb L, Levieux D, Hendil KB, Kuehn L, Bureau JP, Ouali A (2002) Development and evaluation of a sandwich ELISA for quantification of the 20S proteasome in human plasma. J Immunol Methods 260:183–193
Gao XW, Zhou XG, Wang RJ, Zheng BZ (1998) Distribution and purification of acetylcholinesterase in cotton bollworm (Hepidoptera noctuidae) (in Chinese). Acta Entomol Sin 41:19–25
Hirahara K, Tatsuta T, Takatori T, et al (2001) Preclinical evaluation of an immunotherapeutic peptide comprising 7 T-cell determinants of Cry j 1 and Cry j 2, the major Japanese cedar pollen allergens. J Allergy Clin Immunol 108:94–100
Huang YH, Liang WQ, Pan AH, Zhou ZA, Huang C, Chen JX, Zhang DB (2003) The production of FaeG, the major subunit of K88 fimbriae, in transgenic tobacco plants and its immunogenicity in mice. Infect Immun 71:5436–5439
Huang YH, Liang WQ, Wang YJ, Zhou ZA, Pan AH, Yang XH, Huang C, Chen JX, Zhang DB (2005a) Immunogenicity of the epitope of the foot-and-mouth disease virus fused with a hepatitis B core protein as expressed in transgenic tobacco. Viral Immunol 18:668–677
Huang Z, Elkin G, Maloney BJ, Beuhner N, Arntzen C.J, Thanavala Y, Mason HS (2005b) Virus-like particle expression and assembly in plants: hepatitis B and Norwalk viruses. Vaccine 23:1851–1858
Huang N, Yang DC (2005) ExpressTec: high-level expression of biopharmaceuticals in cereal grains. In: Knablein J (ed) Modern biopharmaceuticals. WILEY-VCH, Weinheim, pp 931–947
Hui JY, Li GD, Kong YY, Wang Y (1999) Expression and characterization of chimeric hepatitis B surface antigen particles carrying preS epitopes. J Biotechnol 72:49–59
Iwarson S (1995) New approaches to hepatitis A and B vaccines. APMIS 103:321–326
Jiang X, Wang M, Graham DY, Estes MK (1992) Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J Virol 66:6527–6532
Kapusta J, Modelska A, Figlerowicz M, Pniewski T, Letellier M, Lisowa O, Yusibov V, Koprowski H, Plucienniczak A, Legocki AB (1999) A plant-derived edible vaccine against hepatitis B virus. FASEB J 13:1796–1799
Kong Q, Richter L, Yang YF, Arntzen CJ, Mason HS, Thanavala Y (2001) Oral immunization with hepatitis B surface antigen expressed in transgenic plants. Proc Natl Acad Sci USA 98:1539–1544
Lewis DC (1990) Three serotypes of Norwalk-like virus demonstrated by solid-phase immune electron microscopy. J Med Virol 30:77–81
Liu QQ, Zhang JL, Wang ZY, Hong MM, Gu MH (1998) A highly efficient transformation mediated by Agrobacterium in rice. Acta Phytophysiol Sin 24:259–271
Mason HS, Arntzen CJ (1995) Transgenic plants as vaccine production systems. Trends Biotechnol 13:388–392
Mason HS, Lam DMK, Arntzen CJ (1992) Expression of hepatitis B surface antigen in transgenic plants. Proc Natl Acad Sci USA 89:11745–11749
Mason HS, Warzecha H, Mor T, Arntzen CJ (2002) Edible plant vaccines: applications for prophylactic and therapeutic molecular medicine. Trends Mol Med 8:324–329
Milich DR (1997) Immune response to the hepatitis B virus: infection, animal models, vaccination. Viral Hepat 3:63–103
Neurath AR, Kent SB, Strick N, Parker K (1986) Identification and chemical synthesis of a host cell receptor binding site on hepatitis B virus. Cell 46:429–436
Neurath AR, Seto B, Strick N (1989) Antibodies to synthetic peptides from the preS1 region of the hepatitis B virus (HBV) envelope (env) protein are virus-neutralizing and protective. Vaccine 7:234–236
Qian BJ, Shen HF, Xiong JJ, Chen L, Zhang L, Jia JW, Wang Y, Zhang ZC, Yuan Z, Cao KM, Zhang DB (2006) Expression and purification of the synthetic preS1 gene of hepatitis B virus with preferred Escherichia coli codon preference. Protein Expr Purif 48:74–80
Richter L, Thanavala Y, Arntzen1 CJ, Mason HS (2000) Production of hepatitis B surface antigen in transgenic plants for oral immunization. Nat Biotechnol 18:1167–1171
Sambrook J, Fritsch EF, Maniatis J (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Streatfield SJ (2005) Oral hepatitis B vaccine candidates produced and delivered in plant material. Immunol Cell Biol 83:257–262
Streatfield SJ, Major JM, Barker DK, Brooks C, Woodard SL, Horn M, Nikolov ZL, Hood EE, Jilka JM, Howard JA (2002) Development of an edible subunit vaccine in corn against enterotoxigenic strains of Escherichia coli. In Vitro Cell Dev Biol Plant 38:11–17
Tada Y, Utsumi S, Takaiwa F (2003) Foreign gene products can be enhanced by introduction into low storage protein mutants. Plant Biotechnol J 1:411–422
Thanavala Y, Mahoney M, Pal S, Scott A, Richter L, Natarajan N, Goodwin P, Arntzen CJ, Mason HS (2005) Immunogenicity in humans of an edible vaccine for hepatitis B. Proc Natl Acad Sci USA 102:3378–3382
Thanavala Y, Yang YF, Lyons P, Mason HS, Arntzen CJ (1995) Immunogenicity of transgenic plant-derived hepatitis B surface antigen. Proc Natl Acad Sci USA 92:3358–3361
Tiollais P, Pourcel C, Dejean A (1985) The hepatitis B virus. Nature 317:489–495
Valenzuela P, Medina A, Rutter WJ (1982) Synthesis and assembly of hepatitis B virus surface antigen particles in yeast. Nature 298:347–350
Wei MS, Xiang N, Zhang ZF, Zhang CL, Wang JF, Qiu BS, Tian B (1995) Detection of grapevine fanleaf virus and potato virus Y by cDNA probes labeled with biotin. Acta Phytopathol Sin 25:331–334
Weng HB, Pan AH, Yang LT, Zhang CM, Liu ZL, Zhang DB (2004) Estimating transgene copy number by real-time PCR assay using HMG I/Y as an endogenous reference gene in transgenic rapeseed. Plant Mol Biol Rep 22:289–300
Xu X, Li GD, Kong YY, Yang HL, Zhang ZC, Cao HT, Wang Y (1994) A modified hepatitis B virus surface antigen with the receptor binding site for hepatocytes at its C terminus: expression, antigenicity and immunogenicity. J Gen Virol 75: 3673–3677
Yang LT, Ding JY, Zhang CM, Jia JW, Weng HB, Liu WX, Zhang DB (2005) Estimating the copy number of transgenes in transformed rice by real-time quantitative PCR. Plant Cell Rep 23:759–763
Yang JY, Hui JY, Li GD, Wang Y, Yuan HY, Li YY (2000a) Expression of the recombinant hepatitis B virus surface antigen carrying PreS epitopes in Pichia pastoris. Acta Biochim Biophys Sin 32:139–144
Yang JY, Jin J, Kong YY, Wei J, Zhang ZC, Li GD, Wang Y, Yuan HY, Li YY (2000b) Purification and characterization of recombinant hepatitis B virus surface antigen SS1 expressed in Pichia pastoris. Acta Biochim Biophys Sin 32:503–508
Acknowledgments
This work was supported by the National Key Basic Research Developments Program of the Ministry of Science and Technology, People’s Republic of China (2006CB101700) and National Natural Science Foundation of China (30600347).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Qian, B., Shen, H., Liang, W. et al. Immunogenicity of recombinant hepatitis B virus surface antigen fused with preS1 epitopes expressed in rice seeds. Transgenic Res 17, 621–631 (2008). https://doi.org/10.1007/s11248-007-9135-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-007-9135-6