Abstract
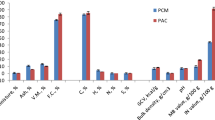
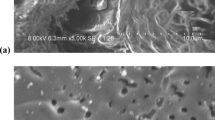
The present study investigated the adsorption efficiency of laboratory-produced activated carbon (AC) from pyrolysis of mix waste plastic (MWP) for treating paper mill effluent. In the present work, adsorbent was prepared from MWP and was characterized in comparison to commercial adsorbents. A lab scale adsorption study was performed in 100 mL batch experiments to get the optimized condition of different variables by eliminating the pollutants from EOP wastewater and was found as AC dose 10.0 g/100 mL, temperature 35 °C, agitation rate 150 rpm, contact time 7 h, and pH 9.0. The EOP wastewater was analyzed for different quality parameters after treating with prepared AC (PW-AC) and two commercial ACs (SC-AC and M-AC) at optimized conditions. The efficiency of ACs was found to be in order as M-AC>PW-AC>SC-AC. The optimized conditions were applied for the removal of chlorophenols using different ACs in comparison to control EOP wastewater. The study revealed that elimination of chlorophenolic compounds from EOP wastewater (7824 ng/L) was the highest in M-AC treatment followed by PW-AC and SC-AC, i.e., 1426 ng/L, 1759 ng/L, and 2200 ng/L, respectively. PW-AC had major impacts on reduction of chloroguaiacols (82%) as well as chlorophenols (80.7%) that were present in chief amount in EOP wastewater. PW-AC was found to be a capable adsorbent for the removal of chlorophenolic compounds from the most toxic bleaching effluents of pulp and paper industry.




Similar content being viewed by others

Data Availability
All data are fully available without restriction.
References
Ali, M., & Sreekrishnan, T. R. (2001). Aquatic toxicity from pulp and paper mill effluents: A review. Advances in Environmental Research, 5, 175–196. https://doi.org/10.1016/S1093-0191(00)00055-1
Antonakou, E. V., Kalogiannis, K. G., Stefanidis, S. D., Karakoulia, S. A., Triantafyllidis, K. S., Lappas, A. A., & Achilias, D. S. (2014). Catalytic and thermal pyrolysis of polycarbonate in a fixed-bed reactor: The effect of catalysts on products yields and composition. Polymer Degradation and Stability, 110, 482–491. https://doi.org/10.1016/j.polymdegradstab.2014.10.007
Bajpai, P. (2001). Microbial degradation of pollutants in pulp mill effluents. https://doi.org/10.1016/S0065-2164(01)48001-4
Berry, R. M., Luthe, C. E., Voss, R. H., Wrist, P. E. (1991). The effects of recent changes in bleached softwood kraft mill technology on organo florine emissions: An internation erspective. Pulp and Paper Canada 92(6).
CEPA (1998). Pentachlorophenol (PCP) risk characterization document, Available at: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwjrsZzjyobrAhUXwjgGHcrIBFYQFjAFegQICBAB&url=https%3A%2F%2Fwww.cdpr.ca.gov%2Fdocs%2Frisk%2Frcd%2Fpentachl.pdf&usg=AOvVaw0BkXd-hPcZfcKRqeUiyzIm [ accessed 02 Sep 2020]
Ciputra, S., Antony, A., Phillips, R., Richardson, D., Leslie, G. (2010) Comparison of treatment options for removal of recalcitrant dissolved organic matter from paper mill effluent. Chemosphere, 86–91 https://doi.org/10.1016/j.chemosphere.2010.06.060
De Sousa Ribeiro, L. A., Thim, G. P., Alvarez-Mendez, M. O., dos Reis Coutinho, A., de Moraes, N. P., & Rodrigues, L. A. (2018). Preparation, characterization, and application of low-cost açaí seed-based activated carbon for phenol adsorption. International Journal of Environmental Research, 12, 755–764. https://doi.org/10.1007/s41742-018-0128-5),-volV)
DWAF (1996). South African water quality guidelines: vol. 4: agricultural use: irrigation (PDF). Department of Water Affairs and Forestry South Africa. 141–153.[ accessed 2 Sep 2020].
Fanchiang, W. L., & Lin, Y. C. (2012). Catalytic fast pyrolysis of furfural over H-ZSM-5 and Zn/H-ZSM-5 catalysts. Applied Catalysis a: General, 419, 102–110. https://doi.org/10.1016/j.apcata.2012.01.017
Feng, Z., Chen, H., Li, H., Yuan, R., Wang, F., Chen, Z., Zhou, B. (2020). Microwave-assisted KOH activated lignite semi-coke for treatment of biologically treated wastewater from pulp and paper mill. Journal of Environmental Chemical Engineering, 103924. https://doi.org/10.1016/j.jece.2020.103924
Fito, J., Abrham, S., & Angassa, K. (2020). Adsorption of methylene blue from textile industrial wastewater onto activated carbon of parthenium hysterophorus. International Journal of Environmental Research, 23, 1–10. https://doi.org/10.1007/s41742-020-00273-2
Ghosh, U. K. (2006). Short sequence environment friendly bleaching of wheat straw pulp.
González, J. F., Román, S., Encinar, J. M., & Martínez, G. (2009). Pyrolysis of various biomass residues and char utilization for the production of activated carbons. Journal of Analytical and Applied Pyrolysis, 85, 134–141. https://doi.org/10.1016/j.jaap.2008.11.035
Gupta, G. K., Liu, H., & Shukla, P. (2019). Pulp and paper industry–based pollutants, their health hazards and environmental risks. Current Opinion in Environmental Science and Health, 12, 48–56. https://doi.org/10.1016/j.coesh.2019.09.010
Gupta, V., Bhardwaj, N. K., Rawal, R.K. (2021). Removal of colour and lignin from paper mill wastewater using activated carbon from plastic mix waste. International Journal of Environmental Science and Technology (2021). https://doi.org/10.1007/s13762-021-03263-9
Hileman, B. (1993). Concerns broaden over chlorine and chlorinated hydrocarbons. Chemical and Engineering News, 11–20.
Hubbe, M. A., Park, J., & Park, S. (2014). Cellulosic substrates for removal of pollutants from aqueous systems: A review. Part 4 dissolved petrochemical compounds. Bioresources, 9, 7782–7925.
Jain, A. K., Gupta, V. K., Jain, S., & Suhas, . (2004). Removal of chlorophenols using industrial wastes. Environmental Science and Technology, 38, 1195–1200. https://doi.org/10.1021/es034412u
Jalil, A. H. (2012). Surface area determination of activated carbons produced from waste tires using adsorption from solution. Tikrit Journal of Pure Science, 17, 99–104.
Kamali, M., Alavi-Borazjani, S. A., Khodaparast, Z., Khalaj, M., Jahanshahi, A., Costa, E., & Capela, I. (2019). Additive and additive-free treatment technologies for pulp and paper mill effluents: Advances, challenges and opportunities. Water Resources and Industry, 21, 100109. https://doi.org/10.1016/j.wri.2019.100109
Kaur, D., Bhardwaj, N. K., & Lohchab, R. K. (2018). Reduction in chlorophenolic compounds during bleaching of rice straw pulp by replacing elemental chlorine with chlorine dioxide. International Journal of Environmental Science and Technology, 15, 1113–1122. https://doi.org/10.1007/s13762-017-1448-2
Kumar, P. (2012). Characterization and removal of chlorinated organics from pulp bleaching effluents (Doctoral dissertation, Ph. D thesis IIT Roorkee).
Liebergott, N., Van Lierop, B., Nolin, A., Faubert, M., & Laflamme, J. (1991). Modifying the bleaching process to decrease AOX formation. Pulp and Paper Canada, 92, 85–89.
Lim, J. W., Zaid, H. F., Isa, M. H., Oh, W. D., Adnan, R., Bashir, M. J., Kiatkittipong, W., & Wang, D. K. (2018). Shielding immobilized biomass cryogel beads with powdered activated carbon for the simultaneous adsorption and biodegradation of 4-chlorophenol. Journal of Cleaner Production, 205, 828–835. https://doi.org/10.1016/j.jclepro.2018.09.153
Mailler, R., Gasperi, J., Coquet, Y., Derome, C., Buleté, A., Vulliet, E., Bressy, A., Varrault, G., Chebbo, G., & Rocher, V. (2016). Removal of emerging micropollutants from wastewater by activated carbon adsorption: Experimental study of different activated carbons and factors influencing the adsorption of micropollutants in wastewater. Journal of Environmental Chemical Engineering, 4(1), 1102–1109.
Manimekalai, T., Sivakumar, N., & Periyasamy, S. (2015). Catalytic pyrolysis of plastic waste into activated carbon and its adsorption studies on acid red 114 as model organic pollutant. International Journal of Chemical Technology Research, 8, 333–348.
Mehmood, K., Rehman, S. K., Wang, J., Farooq, F., Mahmood, Q., Jadoon, A. M., Javed, M. F., & Ahmad, I. (2019). Treatment of pulp and paper industrial effluent using physicochemical process for recycling. Water, 11, 2393. https://doi.org/10.3390/w11112393
Mendoza-Carrasco, R., Cuerda-Correa, E. M., Alexandre-Franco, M. F., Fernández-González, C., & Gómez-Serrano, V. (2016). Preparation of high-quality activated carbon from polyethyleneterephthalate (PET) bottle waste. Its use in the removal of pollutants in aqueous solution. Journal of Environmental Management, 181, 522–535.
Miskolczi, N., Wu, C., Williams, P. T (2017). Pyrolysis of waste plastics using catalysts: Activated carbon, MCM-41 and HZSM-5. International Journal of Chemical Engineering and Applications, 8: 67. https://doi.org/10.18178/ijcea.2017.8.1.632
Mohrig, J. R., Hammond, C. N., & Schatz, P. F. (2006). “Infrared spectroscopy” in techniques in organic chemistry.
Panwar, S., Mishra, S., Endlay, N., Mathur, R. M., & Kulkarni, A. G. (2004). Toxicity reduction of bleach plant effluent by using chemical additives. IPPTA Journal, 16, 45–52.
Prakash, D. (2012). Reduction of toxicity by using chlorine dioxide in paper making. Journal of Science (JOS), 1, 30–35.
Prasetyo, E. N., Rodríguez, R. D., Lukesch, B., Weiss, S., Murkovic, M., Katsoyannos, E., Sygmund, C., Ludwig, R., Nyanhongo, G. S., & Guebitz, G. M. (2015). Laccase–cellobiose dehydrogenase-catalyzed detoxification of phenolic-rich olive processing residues. International Journal of Environmental Science and Technology, 12, 1343–1352. https://doi.org/10.1007/s13762-014-0526-y
Punyapalakul, P., Suksomboon, K., Prarat, P., & Khaodhiar, S. (2013). Effects of surface functional groups and porous structures on adsorption and recovery of perfluorinated compounds by inorganic porous silicas. Se. Sci Technol, 48, 775–788. https://doi.org/10.1080/01496395.2012.710888
Qin, C., Liu, B., Huang, L., Liang, C., Gao, C., & Yao, S. (2018). Adsorptive removal of adsorbable organic halogens by activated carbon. R Soc Open Sci, 5, 181507. https://doi.org/10.1098/rsos.181507
Sharma, N., Bhardwaj, N. K., & Singh, R. B. (2020). Environmental issues of pulp bleaching and prospects of peracetic acid pulp bleaching: A review. Journal of Cleaner Production, 256, 120338. https://doi.org/10.1016/j.jclepro.2020.120338
Savant, D. V., Abdul-Rahman, R., & Ranade, D. R. (2006). Anaerobic degradation of adsorbable organic halides (AOX) from pulp and paper industry wastewater. Bioresource Technology, 97, 1092–1104. https://doi.org/10.1016/j.biortech.2004.12.013
Schut, J. H. (1996). What’s happening to food-grade PET? Plastics World, 54, 42–46.
Singh, M., Pant, M., Godiyal, R. D., & Kumar Sharma, A. (2020). MCDM approach for selection of raw material in pulp and papermaking industry. Materials and Manufacturing Processes, 35, 241–249. https://doi.org/10.1080/10426914.2020.1711917
Sojo, L. E., & Djauhari, J. (1999). Determination of chlorophenolics in waters by membrane solid-phase extraction: Comparison between C18 and activated carbon membranes and between modes of extraction and elution. Journal of Chromatography A, 840, 21–30. https://doi.org/10.1016/S0021-9673(99)00186-7
Sun, Y. B., Joyce, T. W., & Chang, H. M. (1989). Dechlorination and decolorization of high-molecular-weight chlorolignin from bleach plant effluents by an oxidation process. Tappi Journal, 72, 209–213.
Suntio, L. R., Shiu, W. Y., & Mackay, D. (1988). A review of the nature and properties of chemicals present in pulp mill effluents. Chemosphere, 17, 1249–1290. https://doi.org/10.1016/0045-6535(88)90080-X
Teh, C. Y., Budiman, P. M., Shak, K. P., & Wu, T. Y. (2016). Recent advancement of coagulation–flocculation and its application in wastewater treatment. Industrial and Engineering Chemistry, 55, 4363–4389. https://doi.org/10.1021/acs.iecr.5b04703
TERI (2018). Challenges and opportunities plastic waste management in India. Available at: https://www.teriin.org/sites/default/files/2018-06/plastic-waste-management_0.pdf [accessed 15 January 2019].
Thapliyal, B. P., & Tyagi, S. (2015). Water pinch analysis—An innovative approach towards water conservation in pulp and paper industry. IPPTA Journal, 27, 59–66.
Tian, C., Feng, C., Wei, M., & Wu, Y. (2018). Enhanced adsorption of anionic toxic contaminant Congo red by activated carbon with electropositive amine modification. Chemosphere, 208, 476–483.
Vogel, T. M., Criddle, C. S., & McCarty, P. L. (1987). ES&T critical reviews: Transformations of halogenated aliphatic compounds. Environmental Science and Technology, 21, 722–736. https://doi.org/10.1021/es00162a001
Yasuda, H., Yamada, O., Zhang, A., Nakano, K., & Kaiho, M. (2004). Hydrogasification of coal and polyethylene mixture. Fuel, 83, 2251–2254. https://doi.org/10.1016/j.fuel.2004.06.021
Zhang, X., Lei, H., Yadavalli, G., Zhu, L., Wei, Y., & Liu, Y. (2015). Gasoline-range hydrocarbons produced from microwave-induced pyrolysis of low-density polyethylene over ZSM-5. Fuel, 144, 33–42. https://doi.org/10.1016/j.fuel.2014.12.013
Acknowledgements
This study was done in the Avantha Centre for Industrial Research and Development (ACIRD), Yamuna Nagar, Haryana, India. The authors are grateful to Ex-Director, ACIRD for providing the necessary facilities to complete this work and also the Department of Chemistry, Maharishi Markandeshwar Deemed University, Ambala (Haryana), India.
Author information
Authors and Affiliations
Contributions
GV (Ph.D. student) has done all the experiments. RRK (professor) has drafted the manuscript, and BNK is the corresponding author who has supervised the work and prepared all the probable improvements in the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vipul, G., Kant, B. & Kumar, R.R. Remediation of Chlorophenolic Compounds from Paper Mill Effluent Using High-Quality Activated Carbon from Mixed Plastic Waste. Water Air Soil Pollut 232, 326 (2021). https://doi.org/10.1007/s11270-021-05266-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-05266-1