Abstract
The present work highlighted the studies on Cr(VI) reduction by cells of Acinetobacter haemolyticus (A. haemolyticus). The strain tolerated 90 mg Cr(VI) l−1 in LB broth compared to only 30 mg Cr(VI) l−1 in LB agar. From the FTIR analysis, the Cr(III) species formed was also most likely to form complexes with carboxyl, hydroxyl, and amide groups from the bacteria. A TEM study showed the absence of precipitates on the cell wall region of the bacteria. Instead, microprecipitates were observed in the cytoplasmic region of the cells, suggesting the transportation of Cr(VI) into the cells. Intracellular reduction of Cr(VI) was supported by a reductase test using soluble crude cell-free extracts. The specific reductase activity obtained was 0.52 µg Cr(VI) reduced per mg of protein an hour at pH 7.2 and 37°C. Our results indicated that A. haemolyticus can be used as a promising microorganism for Cr(VI) reduction from industrial wastewaters.
Similar content being viewed by others
Introduction
Wastewaters containing Cr(VI) are generated in many industrial processes, including leather-tanning, chrome-plating, stainless steel-welding, pigment production and nuclear weapons generation (Gonzalez et al. 2003). However, due to leakage, poor storage and improper disposal, Cr(VI) has become one of the most frequently detected contaminant at the waste sites (Thacker et al. 2006). Cr(VI) compounds are in general more toxic than Cr(III) compounds due to their stronger oxidizing power (Cervantes et al. 2001; Katz and Salem 1994). From a biological perspective, Cr(III) compounds have very low solubility, thus, restricting their spread and their biological availability (Gonzalez et al. 2003).
Detoxification of Cr(VI) via reduction of Cr(VI) to Cr(III) can be carried out with chemical or biological methods. Conventional methods for the treatment of chromate include chemical reduction using a reducing agent such as sodium sulfite and adsorption on ion exchange or chelating resins. However, these methods consume high amounts of energy and large quantities of chemical reagents which is not economically feasible. Furthermore, the resultant metal-containing chemical sludge can be a potential source of metal pollution. On the other hand, biological methods such as microbiological detoxification of polluted water are economical, safe, and sustainable (Eccles 1995; Shakoori et al. 2000).
A number of chromium resistant bacteria, i.e., Bacillus sp., Leucobacter sp., Exiguobacterium sp. (Sarangi and Krishnan 2008), Pseudomonas sp. (Desai et al. 2008), Brucella sp. (Thacker et al. 2007), Shewanella oneidensis (Daulton et al. 2007) and Achromobacter sp. (Zhu et al. 2008) have reported to reduce Cr(VI). Bacterial reduction of Cr(VI) can be considered as a mechanism of resistance to Cr(VI) (Ohtake et al. 1987). Cr(VI) resistance mechanisms include periplasmic biosorption, intracellular bioaccumulation and biotransformation either via direct enzymatic reaction or indirectly with metabolites (Camargo et al. 2005).
Recently, the potential of Acinetobacter sp. as a Cr(VI) remediation agent has been demonstrated (Srivastava and Thakur 2007; Zakaria et al. 2007). Srivastava and Thakur (2007) reported removal of more than 75% chromium by Acinetobacter sp. after 7 days incubation in a medium containing sodium acetate (0.2% w/v), sodium nitrate (0.1% w/v) and pH 7 (optimized carbon, nitrogen sources and pH). The findings by Zakaria et al. (2007) also gave early indication for a potential industrial application of A. haemolyticus. However, exploring the metal-microbe interaction is a prerequisite for designing effective and efficient remediation strategies and biotechnology treatment facilities (Boyanov et al. 2003).
The present work elucidates the mechanisms of Cr(VI) resistance and removal by Acinetobacter haemolyticus via instrumental analysis, hence its novelty. Preliminary studies were carried out to evaluate the Cr(VI) tolerance and reduction capacity of the isolate. FTIR was used to determine the functional groups involved in the interaction with chromium. FESEM-EDX and TEM analyses were carried out to determine the localization of chromium. The Cr(VI) reductase activity in a soluble crude cell-free extract (CFE) of the bacteria was also investigated. These results provide a greater understanding of Cr(VI) resistance mechanisms by Acinetobacter haemolyticus and aid in the further development of the microorganism for bioremediation of chromium from industrial wastewater.
Materials and methods
Bacterial strain
Acinetobacter haemolyticus (A. haemolyticus), GenBank Acc. No. EF369508, was isolated from Cr(VI)-containing textile dye effluent. This strain has been demonstrated as being able to reduce Cr(VI) by Zakaria et al. (2007).
Growth media
Three growth media were used in this study (in 1 l of distilled deionized water, DDI): LB broth [10 g of tryptone (Oxoid), 5 g of yeast extract (Merck) and 10 g of NaCl (Riedel-de-Haën)], LB agar [10 g of tryptone, 5 g of yeast extract, 10 g of NaCl and 13 g of agar (Merck)] and MS broth [0.03 g NH4C1 (Riedel-de-Haën), 0.03 g K2HPO4 (BDH), 0.05 g KH2PO4 (Univar), 0.01 g NaC1 and 0.01 g MgSO4 · 7H20 (Merck) (Wang and Xiao 1995)]. All media were autoclaved at 120°C for 15 min and stored at room temperature prior to use.
Determination of Cr(VI) tolerance
The Cr(VI) tolerance of A. haemolyticus was evaluated using repli-plate, agar and shake-flask techniques. LB broth was dispensed into the repli-plate dish (Sterilin, USA) followed by Cr(VI) from a stock solution (1,000 mg l−1) to give a Cr(VI) concentration between 5 and 150 mg l−1. A 5% (v/v) inoculum of mid-exponential phase cultures was then added to the repli-plate and incubated at 30°C for 7 days (Memmert, USA). Bacterial growth was measured at OD600 (Genesys 20 Thermospectronic, USA). LB broth with Cr(VI) but without bacterial cell served as control. Cr(VI) tolerance was also evaluated in LB agar where freshly prepared LB agar supplemented with 30–60 mg Cr(VI) l−1 was inoculated with 0.1 ml of a mid-exponential phase culture. Growth on LB agar plates without Cr(VI) served as the control. The cultures were incubated at 30°C for 24 h. Cr(VI) tolerance was measured as the number of colonies formed after the incubation period. For the shake-flask technique, overnight cultures of A. haemolyticus (10%, v/v) were inoculated into 2 l conical flask containing 200 ml LB broth added with 30 and 60 mg Cr(VI) l−1, respectively, from a stock solution. The samples were incubated at 30°C, 200 rpm for 24 h. Three millilitre aliquots were withdrawn at regular time intervals and analyzed for growth at OD 600 nm. A similar experimental setup without bacterial cells served as a control. Stock chromium solutions (1,000 mg l−1) were prepared as follows; 2.829 g K2Cr2O7 (294.18 g mol−1, BDH-GPR™) and 5.124 g CrCl3·6H2O (266.45 g mol−1, Fluka), respectively, were dissolved in 1 l of DDI water. The solutions were filter-sterilized using a 0.2 μm Whatman filter paper.
Total chromium analysis
Total chromium concentrations were determined using atomic absorption spectrophotometry (AAS, Perkin–Elmer AAnalyst 400) at λ = 357.9 nm with a detection limit of 0.1 mg l−1. The Cr(III) content in liquid solution was obtained by subtracting the content of Cr(VI) from that of total chromium.
Interaction between A. haemolyticus-Cr(VI) during Cr(VI) reduction
FESEM-EDX and TEM analysis
Cells of A. haemolyticus were incubated for 24 h on LB agar plates containing 30 mg Cr(VI) l−1. The bacterial colonies formed were then pooled and fixed in 2.5% (v/v) glutaraldehyde (Sigma, USA) in DDI water for 1–2 h at room temperature. Fixed cells were washed twice with DDI water, and post-fixed with 2% (v/v) osmium tetroxide (Fluka, Switzerland) for 1 h. The pellets were subsequently dehydrated using 10–100% (v/v) ethanol for 5 min each and left to dry overnight in a desiccator. The specimens were mounted onto the sample holder with carbon-conductive adhesive tapes and coated with platinum using a sputter coater (Auto fine coater JFC-1600, JEOL) prior to viewing using a field-emission scanning electron microscope (FESEM JSN-670 1F, JEOL) fitted with EDX analysis.
A similar experimental setup was used for TEM analysis followed by 2 min washes in 100% (v/v) acetone after ethanol washing. The cell pellets were then embedded in 50 and 100% (v/v) of epoxy resin (Ted-Pella, California) in acetone for 15 min each. Then, the cell pellets were infiltrated a second time with fresh 100% (v/v) epoxy resin and cured at 60°C overnight. Specimens of 90 nm thickness were sectioned from the embedded blocks using a Leica UltraCut UCT ultramicrotome and mounted on 200-mesh copper TEM grids. The specimens were stained with uranyl acetate (Ted-Pella, California) and post-stained with lead citrate (Ted-Pella, California) for 5 min each. The samples were viewed using a transmission electron microscope (Tecnai G2, Philips), operated at 80 kV.
FTIR
Infrared spectra for A. haemolyticus cells grown for 24 h in LB broth supplemented with (30, 60, and 100 mg Cr(VI) l−1) and without Cr(VI) for 24 h were obtained using a Fourier Transform Infrared Spectrometer (FTIR 1600, Perkin–Elmer). Bacterial cell suspensions were pelleted by centrifugation at 7,000 rpm, 4°C for 30 min. The supernatant obtained was discarded while the pellet was washed in subsequent changes of 0.85% (v/v) saline and DDI water prior to air-drying at 50°C for 8 h (Kamnev et al. 1997). The dried pellet was ground with 200 mg of KBr (spectroscopic grade) in a mortar before pressed into 10 mm diameter disks under 6 tones of pressure before FTIR analysis. The analysis conditions used were 16 scans at a resolution of 4 cm−1 measured between 400–4,000 cm−1.
Crude cell-free extract Cr(VI) reductase test
Preparation of crude CFE
The crude CFE was prepared based on the methods by Thacker et al. (2006, 2007). Briefly, a mid-exponential phase culture, grown in 200 ml LB broth, was harvested by centrifugation at 6,000 g for 20 min at 4°C, washed twice with 20 ml of 10 mM Tris–HCl buffer, pH 7.2 and resuspended in 30 ml of the same buffer. Cells were disrupted by sonication (VibraCell, Sonic and Materials Inc.) for 20 min in cold condition. The resultant homogenate was centrifuged at 8,000 g for 30 min at 4°C and the supernatant (CFE) was used for chromate reductase assays. Protein estimation was carried out using the method by Lowry et al. (1951). The supernatant obtained after harvesting the cells was also filter sterilized and used for chromate reductase assay.
Cr(VI) reductase assay
The methods by McLean and Beveridge (2001) and Pal and Paul (2004) were adopted for Cr(VI) reductase assays. Cr(VI) reduction assays using the crude CFE or supernatant (10 ml) were conducted at 30°C with agitation at 120 rpm in Erlenmeyer flasks containing 5 mg Cr(VI) l−1 under aerobic condition. LB broth without Cr(VI) and autoclaved crude CFE served as controls. Each treatment was done in duplicate and samples were collected at regular time interval for 8 h. Reactions were stopped by heating the samples in hot water bath at 80°C for 1 h immediately after collection. Cr(VI) was determined according to the method described by Thacker et al. (2006).
Results and discussion
Growth and Cr(VI) tolerance of A. haemolyticus
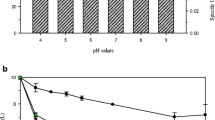
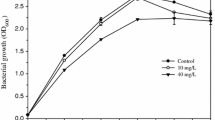
Growth of A. haemolyticus in LB broth was not affected at 30 and 60 mg Cr(VI) l−1, respectively. However, the growth rate decreased from 0.33 (in the absence of Cr(VI) to 0.18 h−1 after the addition of 60 mg Cr(VI) l−1. A. haemolyticus was able to tolerate up to 90 mg Cr(VI) l−1 in LB broth based on a minimum growth percentage of 80%. However, the bacterial growth percentage dropped to 48% at 110 mg Cr(VI) l−1 (Fig. 1) indicating the apparent Cr(VI) toxicity.
One possible mechanism for Cr(VI) toxicity is the alteration of genetic material and altered metabolic and physiological reactions of the bacteria (Losi et al. 1994). However, the use of the repli-plate technique may have contributed to the low Cr(VI) tolerance level due to limited O2 diffusion, hence would ultimately affect the metabolic activity of A. haemolyticus, which is an obligate aerobe (Zakaria et al. 2007). A. haemolyticus exhibited a significantly lower Cr(VI) tolerance in LB agar than in LB broth. A high bacterial count (4.6 × 107 CFU ml−1) was determined at concentrations up to 30 mg Cr(VI) l−1. However, growth was completely inhibited at 40 mg Cr(VI) l−1. A lower Cr(VI) tolerance for A. haemolyticus in solid media may be due to diffusion, complexation and availability of metals (Hassen et al. 1998). In a study by Megharaj et al. (2003), Bacillus sp. and Arthrobacter sp. grew in tryptic soy broth or minimal salts medium-glucose agar added with 100 mg Cr(VI) l−1. Although both bacterial strains exhibited similar resistance on agar media supplemented with Cr(VI), Bacillus sp. did not grow (bacteriostatic) in liquid medium supplemented with 100 mg Cr(VI) l−1. The use of nutrient-rich media may have affected the actual Cr(VI) toxicity via a ‘masking’ effect (Desai et al. 2008; Caravelli et al. 2008). Chelation to various components of the media, such as tryptone and yeast extract, together with the formation of complexes may cause a reduction in the availabilities of free Cr(VI) ions. Of particular importance is the sorption or chelation of metals to unspecified organic compounds found in most biological media (Angle and Chaney 1989).
FESEM-EDX
FESEM micrographs for A. haemolyticus grown with and without Cr(VI) on LB agar plates are presented in Fig. 2. A. haemolyticus showed the following morphology when grown in the absence of Cr(VI); coccobacilli, rough surface and an average cell diameter of 0.577 μm (n = 3) (Figs. 2a, b). However, upon cultivation in 30 mg Cr(VI) l−1 for 24 h, the cells lost their shape and became irregular with appearance of wrinkles on the surface (Figs. 2c, d). The average cell diameter also increased to 0.704 μm (n = 3). Enlargement or elongation of cells were also observed for other bacteria exposed to Cr(VI). Non-adapted E. coli K-12 cells exposed to 250 M potassium chromate (K2CrO4) exhibited extreme filamentous morphology within 3 h of Cr(VI) challenge (Ackerley et al. 2006). Interestingly, numerous pilus-like structures were also observed on A. haemolyticus upon exposure to Cr(VI). A similar observation was reported for Shewanella oneidensis MR-1 where the structures appeared during the transition of growth from 3 to 22°C. these structures were proposed to be sex pili, involved in genetic or nutrients exchange (Abboud et al. 2005).
A ‘wrinkled’ cell surface similar to that of A. haemolyticus upon growing on agar plates containing Cr(VI) has also been reported for another Gram negative bacterium, namely Arthrobacter K-2 (Lin et al. 2006). Acinetobacter sp. was also reported to undergo morphological changes upon contact with Cr(VI) such as the appearance of distinct ridges in the cell wall region (Srivastava and Thakur 2007). However, in this study, no microstructures resembling chromium precipitates were observed on the cell surface of A. haemolyticus implying that chromium was most likely adsorbed onto the surface of A. haemolyticus instead of forming precipitates. The adsorbed chromium is anticipated to be in the Cr(III) form due to the inability of chromate anions to bind with the electronegative functional groups commonly found on gram-negative envelopes (McLean and Beveridge 2001). From the EDX analysis (Fig. 3), small chromium peaks (0.19% wt. analysis) were observed for cells grown with Cr(VI) as opposed to those without Cr(VI). The main elements that co-localized with chromium were typically carbon, oxygen, nitrogen, phosphorus, and sulphur (Niftrik et al. 2008).
TEM
To locate the intracellular accumulation of chromium, TEM analysis was carried out. A. haemolyticus grown on LB agar with Cr(VI) revealed the presence of electron opaque particles that were located mostly in the cytoplasmic region with minimum precipitation on the cell envelopes. This pattern was not found in thin sections of A. haemolyticus grown without Cr(VI) (Fig. 4). A similar finding was also reported for Acinetobacter sp. strain PCP3 by Srivastava and Thakur (2007).
It is tempting to speculate that the electron-opaque particles are attributable to chromium precipitations. We propose that upon entering the cell, Cr(VI) will be reduced to Cr(III) due to the reducing environment and enzymes present inside the cell (Srivastava and Thakur 2007). Subsequently, Cr(III) formed would be free to bind to ionic sites and, once bound, will act as a template for further heterogeneous nucleation and crystal growth. However, long term accumulation of chromium by bacteria can lead to deleterious effect on growth as reported for Shewanella sp. strain MR-4 (Bencheikh-Latmani et al. 2007). Another factor affecting chromium accumulation by A. haemolyticus is the type of growth medium used. A higher chromium accumulation was observed when solid medium was used (as in this study) compared to the use of liquid medium such as nutrient broth. It was noted that in solid medium, the electron-opaque particles were denser (indicating higher chromium accumulation) as compared to the formation of fine particles when A. haemolyticus was grown in nutrient broth with Cr(VI) (Zakaria et al. 2007), which suggested a higher chromium uptake by cells in the solid medium.
FTIR
The FTIR spectra of A. haemolyticus grown in LB broth with and without Cr(VI is shown in Fig. 5.
FTIR spectra of A. haemolyticus grown in LB broth without Cr(VI) suggests the presence of amino, carboxyl, hydroxyl and sulphonate groups with the following band characteristics; 3,400–3,290 cm−1 (broad) represents the –OH and –NH stretching groups, most probably from glucose and proteins, 1,540–1,640 cm−1—primary and secondary amides from the peptide bonds which correspond to –NH bending, 1,100–1,000 cm−1 due to the C–O bond, which is the characteristic peak for polysaccharides, 800–850 cm−1—sulphonate group on the cell surface (Das and Guha 2007). Phosphate functional groups such as P–O, orthophosphate (PO4 3−), and P–OH have characteristic absorption peaks at 1,150, 1,100–1,030, and 1,040–910 cm−1, respectively. However, the peaks occur around the same position as C–N stretching which is between 1,350–1,000 cm−1 (Mungasavalli et al. 2007). When A. haemolyticus was grown in 30, 60, and 100 mg Cr(VI) l−1 (Fig. 5b–d), changes were observed in the region of 1,655–750 and 3,450–2,800 cm−1. These changes indicate the metal binding process taking place on the surface of the cells involving certain functional groups (Bueno et al. 2008) such as amino, carboxyl and hydroxyl groups (Table 1). Similar findings were reported for chromium-treated cyanobacteria where significant alterations were observed for the characteristic peaks of COOH− groups. This observation implies chromium complexation with protein molecules (Pandi et al. 2007; Mungasavalli et al. 2007).
Crude Cr(VI) reductase test
The specific activity for crude CFE of A. haemolyticus was 0.52 μg Cr(VI) reduced per mg of protein an hour at pH 7.2 and 37°C. Percentage of Cr(VI) reduction for both the supernatants and crude CFE (relative to LB broth and autoclaved crude CFE) is shown in Table 2.
The autoclaved crude CFE and the bacterial growth supernatant showed negligible Cr(VI) reductase activity, indicating that that Cr(VI) reductase activity is not associated extracellularly but with the soluble fraction of the cells. The enzyme responsible could be either cytoplasmic or periplasmic-based (Thacker et al. 2006; McLean and Beveridge 2001). The specific activity of the cell fractions in other Cr(VI)-reducing bacteria is shown in Table 3.
Aerobic Cr(VI) reduction is commonly associated with soluble chromate reductases that uses external electron donors such as NADH, NADPH or lactate (Cervantes and Campos-Garcia 2007). NADH, that can also act as cofactors, was also reported to increase the Cr(VI) reduction activity by CFE for a number of bacteria such as Providencia sp. (Thacker et al. 2006), Pseudomonas ambigua G-1 (Suzuki et al. 1992), and E. coli ATCC 33456 (Shen and Wang 1993). However, some strains such as Cellulomonas sp. ES6 showed Cr(VI) reduction activity without external electron donors, suggesting endogenous electron donors for Cr(VI) reduction (Viamajala et al. 2007). Since the CFE of A. haemolyticus also showed substantial Cr(VI) reduction without the addition of external electron donors, it is proposed that A. haemolyticus can also use endogenous electron donors for Cr(VI) reduction.
Conclusion
This study demonstrated that A. haemolyticus, which showed Cr(VI) tolerance and reducing ability, may be used as a potential agent to decontaminate Cr(VI) contamination in wastewater systems. This approach looks promising based on our analysis using FTIR, SEM-EDX, TEM, and Cr(VI) reductase assay where chromium species were demonstrated to interact with the bacterium during the Cr(VI) reduction assay. The Cr(VI) reductase activity was associated with the intracellular soluble fraction of the cells.
References
Abboud R, Popa R, Souza-Egipsy V, Giometti CS, Tollaksen S, Mosher JJ, Findlay RH, Nealson KH et al (2005) Low-temperature growth of Shewanella oneidensis MR-1. Appl Environ Microbiol 71:811–816. doi:10.1128/AEM.71.2.811-816.2005
Ackerley DF, Barak Y, Lynch SV, Curtin J, Matin A et al (2006) Effect of chromate stress on Escherichia coli K-12. J Bacteriol 188:3371–3381. doi:10.1128/JB.188.9.3371-3381.2006
Angle JS, Chaney RL (1989) Cadmium resistance screening in nitrilotriacetate-buffered minimal media. Appl Environ Microbiol 55:2101–2104
Bencheikh-Latmani R, Obraztsova A, Mackey M, Ellisman M, Tebo B et al (2007) Toxicity of Cr(III) to Shewanella sp. strain MR-4 during Cr(VI) reduction. Environ Sci Technol 41:214–220. doi:10.1021/es0622655
Boyanov MI, Kelly SD, Kemner KM, Bunker BA, Fein JB, Fowles DA et al (2003) Adsorption of cadmium to Bacillus subtilis bacterial cell walls: a pH-dependent x-ray absorption fine structure spectroscopy study. Geochim Cosmochim Acta 67:3299–3311. doi:10.1016/S0016-7037(02)01343-1
Bueno BYM, Toremd ML, Molina F, Mesquita LMS et al (2008) Biosorption of lead(II), chromium(III), and copper(II) by R. opacus: equilibrium and kinetic studies. Miner Eng 21:65–75. doi:10.1016/j.mineng.2007.08.013
Camargo FAO, Okeke BC, Bento FM, Frankenberger WY et al (2005) Diversity of chromium-resistant bacteria isolated from soils contaminated with dichromate. Appl Soil Ecol 29:193–202. doi:10.1016/j.apsoil.2004.10.006
Caravelli AH, Giannuzzi L, Zaritzky NE et al (2008) Reduction of hexavalent chromium by Sphaerotilus natans a filamentous micro-organism present in activated sludges. J Hazard Mater 156:214–222. doi:10.1016/j.jhazmat.2007.12.014
Cervantes C, Campos-Garcia J (2007) Reduction and efflux of chromate by bacteria. Mol Microb Heavy Metals Springer-Verlag, Berlin
Cervantes C, Campos-García J, Devars S, Gutiérrez-Corona F, Loza-Tavera H, Torres-Guzmán JC, Moreno-Sánchez R et al (2001) Interactions of chromium with microorganisms and plants. FEMS Microbiol Rev 25:335–347. doi:10.1111/j.1574-6976.2001.tb00581.x
Das SK, Guha AK (2007) Biosorption of chromium by Termitomyces clypeatus. Colloids Surf B Biointerfaces 60:46–54. doi:10.1016/j.colsurfb.2007.05.021
Daulton TL, Little BJ, Jones-Meehan J, Blom DA, Allard LF et al (2007) Microbial reduction of chromium from the hexavalent to divalent state. Geochim Cosmochim Acta 71:556–565. doi:10.1016/j.gca.2006.10.007
Desai C, Jain K, Madamwar D et al (2008) Evaluation of in vitro Cr(VI) reduction potential in cytosolic extracts of three indigeneous Bacillus sp. isolated from Cr(VI) polluted industrial landfill. Bioresour Technol 99:6059–6069
Eccles H (1995) Removal of heavy metals from effluent streams: why select a biological process? Int Biodeterior Biodegradation 35:5–16. doi:10.1016/0964-8305(95)00044-6
Gonzalez CF, Ackerley DF, Park CH, Matin A et al (2003) A soluble flavoprotein contributes to chromate reduction and tolerance by Pseudomonas putida. Acta Biotechnol 2–3:233–239. doi:10.1002/abio.200390030
Hassen A, Saidi N, Cherif M, Boudabous A et al (1998) Resistance of environmental bacteria to heavy metals. Bioresour Technol 64:7–15. doi:10.1016/S0960-8524(97)00161-2
Kamnev AA, Ristić M, Antonyuka LP, Chernyshev AV, Ignatov VV et al (1997) Fourier transform infrared spectroscopic study of intact cells of the nitrogen-fixing bacterium Azospirillum brasdense. J Mol Struct 408/409:201–205. doi:10.1016/S0022-2860(96)09532-4
Katz SA, Salem H (1994) The biological and chemistry of environmental chromium. VCH Publishers, USA
Lameiras S, Quintelas C, Tavares T (2008) Biosorption of Cr(VI) using a bacterial biofilm supported on granular activated carbon and on zeolite. Bioresour Technol 99:801–806. doi:10.1016/j.biortech.2007.01.040
Lin Z, Zhu Y, Kalabegishvili TL, Tsibakhashvili NY, Holman HY et al (2006) Effect of chromate action on morphology of basalt-inhabiting bacteria. Mater Sci Eng 26:610–612. doi:10.1016/j.msec.2005.06.058
Losi ME, Amrhein C, Frankenberger WT et al (1994) Environmental biochemistry of chromium. Rev Environ Contam Toxicol 36:91–121
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ et al (1951) Protein measurements with Folin phenol reagents. J Biol Chem 193:265–275
McLean J, Beveridge TJ (2001) Chromate reduction by a Pseudomonad isolated from a site contaminated with chromated copper arsenate. Appl Environ Microbiol 67:1076–1084. doi:10.1128/AEM.67.3.1076-1084.2001
Megharaj M, Avudainayagam S, Naidu R et al (2003) Toxicity of hexavalent chromium and its reduction by bacteria isolated from soil contaminated with tannery waste. Curr Microbiol 47:51–54. doi:10.1007/s00284-002-3889-0
Mungasavalli DP, Viraraghavan T, Jin YC et al (2007) Biosorption of chromium from aqueous solutions by pretreated Aspergillus niger: batch and column studies. Colloids Surf A Physicochem Eng Asp 301:214–223. doi:10.1016/j.colsurfa.2006.12.060
Niftrik LV, Geerts WJC, Donselaar EGV, Humbel BM, Yakushevska A, Verkleij AJ, Jetten MSM, Strous M et al (2008) Combined structural and chemical analysis of the anammoxosome: a membrane-bounded intracytoplasmic compartment in anammox bacteria. J Struct Biol 161:401–410. doi:10.1016/j.jsb.2007.05.005
Ohtake H, Cervantes C, Silver S et al (1987) Decreased chromate uptake in Pseudomonas fluorescens carrying a chromate resistant plasmid. J Bacteriol 169:3853–3856
Pal A, Paul AK (2004) Aerobic chromate reduction by chromium-resistant bacteria isolated from serpentine soil. Microbiol Res 159:347–354. doi:10.1016/j.micres.2004.08.001
Pandi M, Shashirekha V, Swamy M et al. (2007) Bioabsorption of chromium from retan chrome liquor by cyanobacteria (in press)
Park D, Yun YS, Park JM et al (2005) Studies on hexavalent chromium biosorption by chemically-treated biomass of Ecklonia sp. Chemosphere 60:1356–1364. doi:10.1016/j.chemosphere.2005.02.020
Sarangi A, Krishnan C (2008) Comparison of in vitro Cr(VI) reduction by CFEs of chromate resistant bacteria isolated from chromate contaminated soil. Bioresour Technol 99(10):4130–4137
Shakoori AR, Makhdoom M, Haq RU et al (2000) Hexavalent chromium reduction by a dichromate-resistant gram-positive bacterium isolated from effluents of tanneries. Appl Microbiol Biotechnol 53:348–351. doi:10.1007/s002530050033
Shen H, Wang YT (1993) Characterization of enzymatic reduction of hexavalent chromium by Escherichia coli ATCC 33456. Appl Environ Microbiol 59:3771–3777
Srivastava S, Thakur IS (2007) Evaluation of biosorption potency of Acinetobacter sp for removal of hexavalent chromium from tannery effluent. Biodegradation 18:637–646. doi:10.1007/s10532-006-9096-0
Suzuki T, Miyata N, Horitsu H, Kawai K, Takamizawa K, Tai Y, Okazaki M et al (1992) NAD(P)H-dependent chromium(VI) reductase of Pseudomonas ambigua G-1: a Cr(V) intermediate is formed during the reduction of Cr(VI) to Cr(III). J Bacteriol 174:5340–5345
Thacker U, Parikh R, Shouche Y, Madamwar D et al (2006) Hexavalent chromium reduction by Providencia sp. Process Biochem 41:1332–1337. doi:10.1016/j.procbio.2006.01.006
Thacker U, Parikh R, Shouche Y, Madamwar D et al (2007) Reduction of chromate by cell-free extract of Brucella sp. isolated from Cr(VI) contaminated sites. Bioresour Technol 98:1541–1547. doi:10.1016/j.biortech.2006.06.011
Viamajala S, Smith WA, Sani RK, Apel WA, Petersen JN, Neal AL, Roberto FF, Newby DT, Peyton BM et al (2007) Isolation and characterization of Cr(VI)-reducing Cellulomonas spp from subsurface soils: implications for long-term chromate reduction. Bioresour Technol 98:612–622. doi:10.1016/j.biortech.2006.02.023
Wang YT, Xiao C (1995) Factors affecting hexavalent chromium reduction in pure cultures of bacteria. Water Res 29:2467–2474. doi:10.1016/0043-1354(95)00093-Z
Zakaria ZA, Zakaria Z, Surif S, Ahmad WA et al (2007) Hexavalent chromium reduction by Acinetobacter haemolyticus isolated from heavy metal-contaminated wastewater. J Hazard Mater 146:30–38. doi:10.1016/j.jhazmat.2006.11.052
Zhu W, Chai L, Ma Z, Wang Y, Xiao H, Zhao K, et al (2008) Anaerobic reduction of hexavalent chromium by bacterial cells of Achromobacter sp. strain Ch1. Microbiol Res 163(6):616–623
Acknowledgments
The authors acknowledge the contributions by Puan Aida Suhana bt. Rosly from the Electron Microscopy unit, Institute for Medical Research, Kuala Lumpur for invaluable help with the preparation of specimens for TEM analysis. Our sincere gratitude to the Ministry of Higher Education, Malaysia for funding of the project (FRGS) and the Ministry of Science, Technology and Innovation, Malaysia for the National Science Fellowship scholarship to Quek Hsiao Pei and the Postdoctoral Fellowship to Zainul Akmar Zakaria. Also to Dr. Peter Klappa, Department of Biochemistry, University Of Birmingham for proofreading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pei, Q.H., Shahir, S., Santhana Raj, A.S. et al. Chromium(VI) resistance and removal by Acinetobacter haemolyticus . World J Microbiol Biotechnol 25, 1085–1093 (2009). https://doi.org/10.1007/s11274-009-9989-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-009-9989-2