Abstract
Purpose
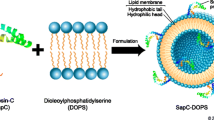
Nanovesicles composed of the phospholipid dioleylphosphatidylserine (DOPS) and a fusogenic protein, saposin C (SapC), selectively target and induce apoptotic cell death in a variety of human cancer cells in vitro and in vivo. We tested whether such tumor-homing nanovesicles are capable of delivering fluorescent probes and magnetic resonance (MR) contrast agents to cancerous tissue to aid in earlier detection and improve visualization.
Procedures
SapC–DOPS nanovesicles labeled with either a far-red fluorescent probe (CellVue® Maroon, CVM) or conjugated with a dextran coated MR contrast agent, ultrasmall superparamagnetic iron oxide (USPIO), were systemically administrated into xenografts for tumor detection using optical and MR imaging systems.
Results
SapC–DOPS nanovesicles were effectively detected in vivo in tumor-bearing animals using both optical and MR imaging techniques, thereby demonstrating the cancer-selective properties of these nanovesicles.
Conclusions
SapC–DOPS nanovesicles offer promise as a new and robust theranostic agent for broad cancer-selective detection, visualization, and potential therapy.






Similar content being viewed by others
References
Hoffman RM, Yang M (2006) Subcellular imaging in the live mouse. Nature Protoc 1:775–782
Marx V (2005) Molecular imaging: companies set out to sharpen the in vivo perspective with new machines and novel contrast agents. Chem Eng News 83:25–34
Fernandes RS, Kirszberg C, Rumjanek VM et al (2006) On the molecular mechanisms for the highly procoagulant pattern of C6 glioma cells. J Thromb Haemost 4:1546–52
Ran S, Downes A, Thorpe PE (2002) Increased exposure of anionic phospholipids on the surface of tumor blood vessels. Cancer Res 62:6132–40
Ran S, Thorpe PE (2002) Phosphatidylserine is a marker of tumor vasculature and a potential target for cancer imaging and therapy. Int J Radiat Oncol Biol Phys 54:1479–84
Utsugi T, Schroit AJ, Connor J et al (1991) Elevated expression of phosphatidylserine in the outer membrane leaflet of human tumor cells and recognition by activated human blood monocytes. Cancer Res 51:3062–6
Qi X, Chu Z (2004) Fusogenic domain and lysines in saposin C. Arch Biochem Biophys 424:210–8
Sandhoff K, Kolter T, Harzer K (2000) Sphingolipid activator proteins. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease, 8th edn. McGraw-Hill, New York, pp 3371–3388
Nieh MP, Pencer J, Katsaras J et al (2006) Spontaneously forming ellipsoidal phospholipid unilamellar vesicles and their interactions with helical domains of saposin C. Langmuir 22:11028–33
Wang Y, Grabowski GA, Qi X (2003) Phospholipid vesicle fusion induced by saposin C. Arch Biochem Biophys 415:43–53
Qi X, Chu Z, Mahller YY et al (2009) Cancer-selective targeting and cytotoxicity by liposomal-coupled lysosomal saposin C protein. Clin Cancer Res 15:5840–5851
Sachdeva MS (1998) Drug targeting systems for cancer chemotherapy. Expert Opin Investig Drugs 7:1849–64
Stark DD, Weissleder R, Elizondo G et al (1998) Superparamagnetic iron oxide: clinical application as a contrast agent for MR imaging of the liver. Radiology 168:297–301
Bulte JW, Kraitchman DL (2004) Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed 17:484–499
Mulder WJ, Strijkers GJ, van Tilborg GA et al (2006) Lipid-based nanoparticles for contrast-enhanced MRI and molecular imaging. NMR Biomed 19:142–164
Dutton AH, Tokuyasu KT, Singer SJ (1979) Iron-dextran antibody conjugates: general method for simultaneous staining of two components in high-resolution immunoelectron microscopy. Proc Natl Acad Sci USA 76:3392–3396
Renshaw PF, Owen CS, Evans AE et al (1986) Immunospecific NMR contrast agents. Magn Reson Imaging 4:351–357
Bulte JW, Hoekstra Y, Kamman RL et al (1992) Specific MR imaging of human lymphocytes by monoclonal antibody-guided dextran-magnetite particles. Magn Reson Med 25:148–157
Tiefenauer LX, Tschirky A, Kuhne G et al (1996) In vivo evaluation of magnetite nanoparticles for use as a tumor contrast agent in MRI. Magn Reson Imaging 14:391–402
Bogdanov AA Jr, Martin C, Weissleder R et al (1994) Trapping of dextran-coated colloids in liposomes by transient binding to aminophospholipid: preparation of ferrosomes. Biochim Biophys Acta 1193:212–218
Chu Z, Sun Y, Kuan CY et al (2005) Saposin C: neuronal effect and CNS delivery by liposomes. Ann NY Acad Sci 1053:237–246
Sternberg B (1993) Freeze-fracture electron microscopy of liposomes. In: Gregoriadis G (ed) Liposome Technology, Vol 1. CRC Press, Boca Raton, pp 363–374
Frank JA, Zywicke H, Jordan EK et al (2002) Magnetic intracellular labeling of mammalian cells by combining (FDA-approved) superparamagnetic iron oxide MR contrast agents and commonly used transfection agents. Acad Radiol 9(Suppl 2):S484–S487
Shapiro EM, Skrtic S, Sharer K et al (2004) MRI detection of single particles for cellular imaging. Proc Natl Acad Sci USA 101:10901–10906
Acknowledgments
This work was funded in part by CancerFree Kids as well as Translational Research Initiative and Validation Research Grants from Cincinnati Children’s Medical Center.
Conflict of Interest
Xiaoyang Qi, PhD is listed as an inventor on the patent for the technology (SapC–DOPS) that is the subject of this research. Consistent with current Cincinnati Children’s Hospital Medical Center policies, the development and commercialization of this technology has been licensed to Bexion Pharmaceuticals, LLC, in which Dr. Qi, holds a minor (<5%) equity interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaimal, V., Chu, Z., Mahller, Y.Y. et al. Saposin C Coupled Lipid Nanovesicles Enable Cancer-Selective Optical and Magnetic Resonance Imaging. Mol Imaging Biol 13, 886–897 (2011). https://doi.org/10.1007/s11307-010-0417-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-010-0417-7