Abstract
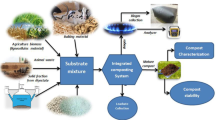
Co-composting biowastes such as manures and biosolids can be used to stabilize carbon (C) without impacting the quality of these biowastes. This study investigated the effect of co-composting biowastes with alkaline materials on C stabilization and monitored the fertilization and revegetation values of these co-composts. The stabilization of C in biowastes (poultry manure and biosolids) was examined by their composting in the presence of various alkaline amendments (lime, fluidized bed boiler ash, flue gas desulphurization gypsum, and red mud) for 6 months in a controlled environment. The effects of co-composting on the biowastes’ properties were assessed for different physical C fractions, microbial biomass C, priming effect, potentially mineralizable nitrogen, bioavailable phosphorus, and revegetation of an urban landfill soil. Co-composting biowastes with alkaline materials increased C stabilization, attributed to interaction with alkaline materials, thereby protecting it from microbial decomposition. The co-composted biowastes also increased the fertility of the landfill soil, thereby enhancing its revegetation potential. Stabilization of biowastes using alkaline materials through co-composting maintains their fertilization value in terms of improving plant growth. The co-composted biowastes also contribute to long-term soil C sequestration and reduction of bioavailability of heavy metals.






Similar content being viewed by others
References
Almaroai YA, Vithanage M, Rajapaksha AU, Lee SS, Doud X, Lee YH, Sung JK, Ok YS (2014) Natural and synthesised iron-rich amendments for As and Pb immobilisation in agricultural soil. Chem Ecol 30:267–279
Andreoli CV, Pegorini ES, Fernandes F, dos Santos HF (2007) Land application of sewage sludge. In: Andreoli CV, von Sperling M, Fernandes F (eds) Sludge treatment and disposal. IWA Publishing, London, pp 162–206
Barje F, El Fels L, El Hajjouji H, Winterton P, Hafidi M (2013) Biodegradation of organic compounds during co-composting of olive oil mill waste and municipal solid waste with added rock phosphate. Environ Technol 34:2965–2975
Bhupinderpal-Singh HMJ, Saggar S, Francis GS (2004) Chemical fractionation to characterize changes in sulphur and carbon in soil caused by management. Eur J Soil Sci 55:79–90
Blagodatskaya E, Kuzyakov Y (2011) Priming effects in relation to soil conditions–Mechanisms. In: Gliński J, Horabik J, Lipiec J (eds) Encyclopedia of Agrophysics. Springer, The Netherlands, pp 657–667
Bloem J, Hopkins DW, Benedetti A (2006) Microbiological methods for assessing soil quality. CABI Publishing, Wallingford, UK
Bolan NS, Kunhikrishnan A, Choppala GK, Thangarajan R, Chung JW (2012) Stabilization of carbon in composts and biochars in relation to carbon sequestration and soil fertility. Sci Total Environ 424:264–270
Bolan NS, Kunhikrishnan A, Naidu R (2013) Carbon storage in a heavy clay soil landfill site after biosolid application. Sci Total Environ 465:216–225
Chevallier T, Woignier T, Toucet J, Blanchart E, Dieudonne P (2008) Fractal structure in natural gels: effect on carbon sequestration in volcanic soils. J Sol-Gel Sci Technol 48:231–238
Chowdhury S (2013) Nutrient amendment and carbon sequestration in soil. PhD Dissertation, University of South Australia, Mawson Lakes, SA, Australia
Chowdhury S, Farrell M, Bolan N (2014) Priming of soil organic carbon by malic acid addition is differentially affected by nutrient availability. Soil Biol Biochem 77:158–169
Chowdhury S, Farrell M, Butler G, Bolan N (2015) Assessing the effect of crop residue removal on soil organic carbon storage and microbial activity in a no-till cropping system. Soil Use Manage. doi:10.1111/sum.12215
Day GM, Hart BT, McKelvie ID, Beckett R (1994) Adsorption of natural organic matter onto goethite. Colloid Surface A 89:1–13
Dinis MAP (2010) Co-composting: a brief review. J Sci Technol College 7:20–30
Fang M, Wong J (1999) Effects of lime amendment on availability of heavy metals and maturation in sewage sludge composting. Environ Pollut 106:83–89
Fang M, Wong JWC, Li GX, Wong MH (1998) Changes in biological parameters during co-compositioning of sewage sludge and coal ash residues. Bioresour Technol 64:55–61
Fang M, Wong JWC, Ma KK, Wong MH (1999) Co-composting of sewage sludge and coal fly ash: nutrient transformations. Bioresour Technol 67:19–24
Farrell M, Jones DL (2009) Critical evaluation of municipal solid waste composting and potential compost markets. Bioresour Technol 100:4301–4310
Farzadkia M, Bazrafshan E (2014) Lime stabilization of waste activated sludge. Health Scope 3:1–4
Fernandez JM, Hockaday WC, Plaza C, Polo A, Hatcher PG (2008) Effects of long-term soil amendment with sewage sludges on soil humic acid thermal and molecular properties. Chemosphere 73:1838–1844
Fortin J, Karam A (2001) Phosphorus sorption by red mud residue as affected by concentration and reaction time. Agrochimica 45:55–66
Gaunt JL, Lehmann J (2008) Energy balance and emissions associated with biochar sequestration and pyrolysis bioenergy production. Environ Sci Technol 42:4152–4158
Gillis JD (2011) Monitoring organic contaminant concentrations and carbon mineralization in field soils receiving alkaline-stabilized biosolids. Master Dissertation, Dalhousie University Halifax, Nova Scotia
Jenkinson DS, Ladd JN (1981) Microbial biomass in soil: measurement and turnover. In: Paul EA, Ladd JN (eds) Soil Biochemistry. Dekker, New York, pp 415–471
Jin VL, Johnson MVV, Haney RL, Arnold JG (2011) Potential carbon and nitrogen mineralization in soils from a perennial forage production system amended with class B biosolids. Agri Ecosyst Environ 141:461–465
Jones BEH, Haynes RJ (2011) Bauxite Processing Residue: a critical review of its formation, properties, storage, and revegetation. Crit Rev Env Sci Tec 41:271–315
Kookana R, Sarmah A, Van Zwieten L, Krull E, Singh B (2011) Biochar application to soil: agronomic and environmental benefits and unintended consequences. Adv Agron 112:103–144
Kuzyakov Y, Friedel JK, Stahr K (2000) Review of mechanisms and quantification of priming effects. Soil Biol Biochem 32:1485–1498
Lamb DT, Heading S, Bolan NS, Naidu R (2012) Use of biosolids for phytocapping of landfill soil. Water Air Soil Pollut 223:2695–2705
Majumder R, Livesley SJ, Gregory D, Arndt SK (2014) Biosolid stockpiles are a significant point source for greenhouse gas emissions. J Environ Manage 143:34–43
Mikutta R, Zang U, Chorover J, Haumaier L, Kalbitz K (2011) Stabilization of extracellular polymeric substances (Bacillus subtilis) by adsorption to and coprecipitation with Al forms. Geochim Cosmochim Acta 75:3135–154
Mulvaney RL (1996) Nitrogen - Inorganic forms. In: Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatabai MA, Johnston CT, Sumner ME (eds) Methods of soil analysis Part 3: chemical methods. Soil Science Society of America Inc, Madison, pp 1123–1184
Murtaza G, Haynes R, Naidu R, Belyaeva O, Kim KR, Lamb D, Bolan N (2011) Natural attenuation of Zn, Cu, Pb and Cd in three biosolids-amended soils of contrasting ph measured using rhizon pore water samplers. Water Air Soil Pollut 221:351–363
Ngo PT, Rumpel C, Ngo QA, Alexis M, Vargas GV, Gil MLM, Dang DK, Jouquet P (2013) Biological and chemical reactivity and phosphorus forms of buffalo manure compost, vermicompost and their mixture with biochar. Bioresour 148:401–407
Novak J, Min PC (2010) The effect of iron and aluminium for phosphorus removal on anaerobic digestion and organic sulfur generation. Water Sci Technol 62:419–426
Olsen SR, Cole C, Watanabe FS, Dean L (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. US Department of Agriculture, Washington DC, pp 1–19
Ro KS, Cantrell KB, Hunt PG (2008) High-temperature pyrolysis of blended animal manures for producing renewable energy and value-added biochar. Ind Eng Chem Res 49:10125–10131
Scheel T, Jansen B, Van Wijk A, Verstraten J, Kalbitz K (2008) Stabilization of dissolved organic matter by aluminium: a toxic effect or stabilization through precipitation? Eur J Soil Sci 59:1122–1132
Seshadri B, Bolan NS, Choppala G, Naidu R (2013) Differential effect of coal combustion products on the bioavailability of phosphorus between inorganic and organic nutrient sources. J Haz Mat 261:817–825
Sikora LJ (2004) Effect of industrial byproducts containing high aluminum or iron levels on plant residue decomposition. Commun Soil Sci Plan 35:921–936
Smolders AJP, Lamers LPM, Moonen M, Zwaga K, Roelofs JGM (2001) Controlling phosphate release from phosphate-enriched sediments by adding various iron compounds. Biogeochemistry 54:219–228
Tandy S, Healey JR, Nason MA, Williamson JC, Jones DL (2009) Heavy metal fractionation during the co-composting of biosolids, deinking paper fibre and green waste. Bioresour Technol 100:4220–4226
Tarrason D, Ojeda G, Ortiz O, Alcaniz JM (2010) Effects of different types of sludge on soil microbial properties: a field experiment on degraded Mediterranean soils. Pedosphere 20:681–691
Thangarajan R, Bolan NS, Tian G, Naidu R, Kunhikrishnan A (2013) Role of organic amendment application on greenhouse gas emission from soil. Sci Total Environ 465:72–96
Torri SI, Correa RS (2012) Downward movement of potentially toxic elements in biosolids amended soils. Appl Environ Soil Sci. doi:10.1155/2012/145724
Vance E, Brookes P, Jenkinson D (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Verma LS, Marschner P (2013) Compost effects on microbial biomass and soil P pools as affected by particle size and soil properties. J Soil Sci Plant Nutr 13:313–328
Wong JWC, Selvam A (2006) Speciation of heavy metals during co-composting of sewage sludge with lime. Chemosphere 63:980–986
Wong JW, Fang M, Jiang R (2001) Persistency of bacterial indicators in biosolids stabilization with coal fly ash and lime. Water Environ Res 73:607–611
Zhang M, Ok YS (2014) Biochar soil amendment for sustainable agriculture with carbon and contaminant sequestration. Carbon Manag 5:255–257
Zimmerman AR (2010) Abiotic and microbial oxidation of laboratory-produced black carbon (biochar). Environ Sci Technol 44:1295–1301
Acknowledgments
This research was supported by the Co-operative Research Centre for Contamination Assessment and Remediation of the Environment (CRC CARE) and Australia Research Council Discovery-Projects (DP140100323). Dr Chowdhury is supported by the Ministry of Environment through ‘The GAIA Project’ in Korea Republic. The postdoctoral fellowship program (PJ010896) at the National Academy of Agricultural Science, Rural Development Administration, Republic of Korea, supported Dr Kunhikrishnan’s contribution.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hailong Wang
Highlights
• Carbon stabilization of biowastes was increased by co-composting with alkaline materials
• The nutrient value of biowastes was not impacted by co-composting
• Priming effect was positively influenced by the labile C of the co-composts
• Co-composted biowastes enhanced potentially mineralizable N and Olsen P in soil
• Co-composted biowastes decreased Cd uptake by Indian mustard
Rights and permissions
About this article
Cite this article
Chowdhury, S., Bolan, N.S., Seshadri, B. et al. Co-composting solid biowastes with alkaline materials to enhance carbon stabilization and revegetation potential. Environ Sci Pollut Res 23, 7099–7110 (2016). https://doi.org/10.1007/s11356-015-5411-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5411-9