Abstract
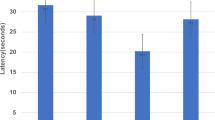
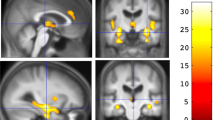
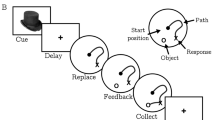
Of the acetylcholine muscarinic receptors, the type 1 (M1) and type 2 (M2) receptors are expressed at the highest levels in the prefrontal cortex (PFC) and hippocampus, brain regions important for cognition. As equivocal findings of age-related changes of M1 and M2 in the nonhuman primate brain have been reported, we first assessed age-related changes in M1 and M2 in the PFC and hippocampus using saturation binding assays. Maximum M1 receptor binding, but not affinity of M1 receptor binding, decreased with age. In contrast, the affinity of M2 receptor binding, but not maximum M2 receptor binding, increased with age. To determine if in the elderly cognitive performance is associated with M1 or M2 function, we assessed muscarinic function in elderly female rhesus macaques in vivo using a scopolamine challenge pharmacological magnetic resonance imaging and in vitro using saturation binding assays. Based on their performance in a spatial maze, the animals were classified as good spatial performers (GSP) or poor spatial performers (PSP). In the hippocampus, but not PFC, the GSP group showed a greater change in T2*-weighted signal intensity after scopolamine challenge than the PSP group. The maximum M1 receptor binding and receptor binding affinity was greater in the GSP than the PSP group, but no group difference was found in M2 receptor binding. Parameters of circadian activity positively correlated with the difference in T2*-weighted signal intensity before and after the challenge, the maximum M1 receptor binding, and the M1 receptor binding affinity. Thus, while in rhesus macaques, there are age-related decreases in M1 and M2 receptor binding, in aged females, hippocampal M1, but not M2, receptor function is associated with spatial learning and memory and circadian activity.




Similar content being viewed by others
References
Ali-Melkkila T, Kanto J, Iisalo E (1993) Pharmacokinetics and related pharmacodynamics of anticholinergic drugs. Acta Anaesthesiol Scand 37(7):633–642
Araujo JA, Studzinski CM, Milgram NW (2005) Further evidence for the cholinergic hypothesis of aging and dementia from the canine model of aging. Prog Neuropsychopharmacol Biol Psychiatry 29(3):411–422
Banta Lavenex P, Lavenex P (2009) Spatial memory and the monkey hippocampus: not all space is created equal. Hippocampus 19(1):8–19
Bartholomeusz CF, Wesnes KA, Kulkarni J, Vitetta L, Croft RJ, Nathan PJ (2008) Estradiol treatment and its interaction with the cholinergic system: effects on cognitive function in healthy young women. Horm Behav 54(5):684–693
Biggan SL, Ingles JL, Beninger RJ (1996) Scopolamine differentially affects memory of 8- and 16-month-old rats in the double y-maze. Neurobiol Aging 17(1):25–30
Cho YH, Friedman E, Silva AJ (1999) Ibotenate lesion of the hippocampus impair spatial learning but not contextual fear conditioning. Behav Brain Res 98:77–87
Costall B, Barnes JM, Hamon M, Muller WE, Briley M (1990) Biochemical models for cognition enhancers. Pharmacopsychiatry 23(Suppl 2):85–88, discussion 89
Day J, Damsma G, Fibiger HC (1991) Cholinergic activity in the rat hippocampus, cortex and striatum correlates with locomotor activity: an in vivo microdialysis study. Pharmacol Biochem Behav 38(4):723–729
Dumas JA, Saykin AJ, McDonald BC, McAllister TW, Hynes ML, Newhouse PA (2008) Nicotinic versus muscarinic blockade alters verbal working memory-related brain activity in older women. Am J Geriatr Psychiatry 16(4):272–282
Ebert U, Kirch W (1998) Scopolamine model of dementia: electroencephalogram findings and cognitive performance. Eur J Clin Investig 28(11):944–949
Ebert U, Grossmann M, Oertel R, Gramatte T, Kirch W (2001) Pharmacokinetic-pharmacodynapic modeling of the electroencephalogram effects of scopolamine in healthy volunteers. J Clin Pharmacol 41:51–60
Eger EI, Zhang Y, Laster M, Food P, Kendig JJ, Sonner JM (2002) Acetylcholine receptors do not mediate the immobilization produced by inhaled anesthetics. Anaesth Analg 94:1500–1504
Elrod K, Buccafusco JJ (1988) An evaluation of the mechanism of scopolamine-induced impairment in two passive avoidance protocols. Pharmacol Biochem Behav 29(1):15–21
Fisher A (2008) Cholinergic treatments with emphasis on M1 muscarinic agonists as potential disease-modifying agents for Alzheimer’s disease. Neurotherapeutics 5(3):433–442
Fisher A, Pittel Z, Haring R, Bar-Ner N, Kliger-Spatz M, Natan N, Egozi I, Sonego H, Marcovitch I, Brandeis R (2003) M1 muscarinic agonists can modulate some of the hallmarks in Alzheimer’s disease: implications in future therapy. J Mol Neurosci 20(3):349–356
Flynn DD, Ferrari-DiLeo G, Levey AI, Mash DC (1995a) Differential alterations in muscarinic receptor subtypes in alzheimer’s disease: implications for cholinergic-based therapies. Life Sci 56(11–12):869–876
Flynn DD, Ferrari-DiLeo G, Mash DC, Levey AI (1995b) Differential regulation of molecular subtypes of muscarinic receptors in Alzheimer’s disease. J Neurochem 64(4):1888–1891
Fransson P (2004) Spontaneous low-frequency bold signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp 26(1):15–29
Fredrickson A, Snyder PJ, Cromer J, Thomas E, Lewis M, Maruff P (2008) The use of effect sizes to characterize the nature of cognitive change in psychopharmacological studies: an example with scopolamine. Hum Psychopharmacol 23(5):425–436
Frey KA, Ehrenkaufer RLE, Beaucage S, Agranoff BW (1985) Quantitative in vivo receptor binding: I. Theory and application to the muscarinic cholinergic receptor. J Neurosci 5(2):421–428
Frey KA, Ciliax B, Agranoff BW (1991) Quantitative in vivo receptor binding: IV. Detection of muscarinic receptor down-regualtion by equilibrium and by tracer kinetic methods. Neurochem Res 16(9):1017–1023
Gage FH, Chen KS, Buzsaki G, Armstrong D (1988) Experimental approaches to age-related cognitive impairments. Neurobiol Aging 9(5–6):645–655
Gautam D, Duttaroy A, Cui Y, Han SJ, Deng C, Seeger T, Alzheimer C, Wess J (2006) M1–M3 muscarinic acetylcholine receptor-deficient mice: novel phenotypes. J Mol Neurosci 30(1–2):157–160
Gillette MU, Buchanan GF, Artinian L, Hamilton SE, Nathanson NM, Liu C (2001) Role of the M1 receptor in regulating circadian rhythms. Life Sci 68(22–23):2467–2472
Gold S, Christian B, Arndt S, Zeien G, Cizadlo T, Johnson DL, Flaum M, Andreasen NC (1998) Functional MRI statistical software packages: a comparative analysis. Hum Brain Mapp 6(2):73–84
Haimov I, Hanuka E, Horowitz Y (2008) Chronic insomnia and cognitive functioning among older adults. Behav Sleep Med 6(1):32–54
Haley GE, Landauer N, Renner L, Weiss A, Hooper K, Urbanski HF, Kohama SG, Neuringer M, Raber J (2009) Circadian activity associated with spatial learning and memory in aging rhesus monkeys. Exp Neurol 217(1):55–62
Hartikainen K, Rorarius M (1999) Cortical responses to auditory stiuli during isoflurane burst suppression. Anaesthesia 54:210–214
Jagoda EM, Kiesewetter DO, Shimoji K, Ravasi L, Yamada M, Gomeza J, Wess J, Eckelman WC (2003) Regional brain uptake of the muscarinic ligand, [18f]fp-tztp, is greatly decreased in M2 receptor knockout mice but not in M1, M3 and M4 receptor knockout mice. Neuropharmacology 44(5):653–661
Klinkenberg I, Blokland A (2010) The validity of scopolamine as a pharmacological model for cognitive impairment: a review of animal behavioral studies. Neurosci Biobehav Rev (in press)
Knutson B, Gibbs SE (2007) Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology (Berl) 191(3):813–822
Lavenex PB, Amaral DG, Lavenex P (2006) Hippocampal lesion prevents spatial relational learning in adult macaque monkeys. J Neurosci 26(17):4546–4558
Liu C, Gillette MU (1996) Cholinergic regulation of the suprachiasmatic nucleus circadian rhythm via a muscarinic mechanism at night. J Neurosci 16(2):744–751
Logothetis NK, Guggenberger H, Peled S, Pauls J (1999) Functional imaging of the monkey brain. Nat Neurosci 2(6):555–562
Luan L, Ding F, Ai Y, Andersen A, Hardy P, Forman E, Gerhardt GA, Gash DM, Grondin R, Zhang Z (2008) Pharmacological MRI (phMRI) monitoring of treatment in hemiparkinsonian rhesus monkeys. Cell Transplant 17(4):417–425
Ma L, Seager MA, Wittmann M, Jacobson M, Bickel D, Burno M, Jones K, Graufelds VK, Xu G, Pearson M, McCampbell A, Gaspar R, Shughrue P, Danziger A, Regan C, Flick R, Pascarella D, Garson S, Doran S, Kreatsoulas C, Veng L, Lindsley CW, Shipe W, Kuduk S, Sur C, Kinney G, Seabrook GR, Ray WJ (2009) Selective activation of the M1 muscarinic acetylcholine receptor achieved by allosteric potentiation. Proc Natl Acad Sci USA 106(37):15950–15955
Matsui M, Yamada S, Oki T, Manabe T, Taketo MM, Ehlert FJ (2004) Functional analysis of muscarinic acetylcholine receptors using knockout mice. Life Sci 75(25):2971–2981
Morin CM, Blais FC, Mimeault V (1998) Sleep disturbances in late life. In: Hersen M, Van Hasselt VB (eds) Handbook of clinical geropsychology. Plenum, New York, pp 273–299
Murrell JC, Waters D, Johnson CB (2008) Comparative effects of halothane, isoflurane, sevoflurane, and desflurane on the electroencephalogram of the rat. Lab Anim 42:161–170
Nakayama T, Penheiter AR, Penheiter SG, Chini EN, Thompson M, Warner DO, Jones KA (2006) Differential effects of volatile anesthetics on M3 muscarinic receptor coupling to the gαq heterotrimeric g protein. Anesthesiology 105:313–324
Nathanson NM (2008) Synthesis, trafficking, and localization of muscarinic acetylcholine receptors. Pharmacol Ther 119(1):33–43
Ogawa T, Shingu K, Shibata M, Osawa M, Mori K (1992) The divergent actions of volatile anaesthetics on background neuronal activity and reactive capability in the central nervous system in cats. Can J Anaesth 39:862–872
Pereira FR, Alessio A, Sercheli MS, Pedro T, Bilevicius E, Rondina JM, Ozelo HF, Castellano G, Covolan RJ, Damasceno BP, Cendes F (2010) Asymmetrical hippocampal connectivity in mesial temporal lobe epilepsy: evidence from resting state fMRI. BMC Neurosci 11:66
Porkkala T, Kaukinen S, Hakkinen V, Jantti V (1997) Median nerve somatosenory evoked potentials during isoflurane anaesthesia. Can J Anaesth 44:963–968
Qiu A, Tuan TA, Woon PS, Abdul-Rahman MF, Graham S, Sim K (2010) Hippocampal-cortical structural connectivity disruptions in schizophrenia: an integrated perspective from hippocampal shape, cortical thickness, and integrity of white matter bundles. NeuroImage (in press)
Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg SR, Fike JR (2004) Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res 162:39–47
Rosier A, Cornette L, Orban GA (1998) Scopolamine-induced impairment of delayed recognition of abstract visual shapes. Neuropsychobiology 37(2):98–103
Rouse ST, Edmunds SM, Yi H, Gilmor ML, Levey AI (2000) Localization of m(2) muscarinic acetylcholine receptor protein in cholinergic and non-cholinergic terminals in rat hippocampus. Neurosci Lett 284(3):182–186
Sarter M, Turchi J (2002) Age- and dementia-associated impairments in divided attention: psychological constructs, animal models, and underlying neuronal mechanisms. Dement Geriatr Cogn Disord 13(1):46–58
Savage UC, Faust WB, Lambert P, Moerschbaecher JM (1996) Effects of scopolamine on learning and memory in monkeys. Psychopharmacology (Berl) 123(1):9–14
Scali C, Vannucchi MG, Pepeu G, Casamenti F (1995) Peripherally injected scopolamine differentially modulates acetylcholine release in vivo in the young and aged rats. Neurosci Lett 197(3):171–174
Small SA, Chawla MK, Buonocore M, Rapp PR, Barnes CA (2004) Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proc Natl Acad Sci USA 101(18):7181–7186
Taffe MA, Weed MR, Gold LH (1999) Scopolamine alters rhesus monkey performance on a novel neuropsychological test battery. Brain Res Cogn Brain Res 8(3):203–212
Tamminga CA (2006) The neurobiology of cognition in schizophrenia. J Clin Psychiatry 67(9):e11
Thathiah A, De Strooper B (2009) G protein-coupled receptors, cholinergic dysfunction, and A beta toxicity in Alzheimer’s disease. Sci Signal 2(93):re8
Thiel CM (2003) Cholinergic modulation of learning and memory in the human brain as detected with functional neuroimaging. Neurobiol Learn Mem 80:234–244
Thomas E, Snyder PJ, Pietrzak RH, Jackson CE, Bednar M, Maruff P (2008) Specific impairments in visuospatial working and short-term memory following low-dose scopolamine challenge in healthy older adults. Neuropsychologia 46(10):2476–2484
Tinkler GP, Voytko ML (2005) Estrogen modulates cognitive and cholinergic processes in surgically menopausal monkeys. Prog Neuropsychopharmacol Biol Psychiatry 29(3):423–431
Tsushima K, Shingu K, Ikeda S, Kimura H, Yamada K, Murao K (1998) Supressive actions of volatile anaesthetics on the response capability in cats. Can J Anaesth 45:240–245
Van Kampen JM, Eckman CB (2009) Agonist-induced restoration of hippocampal neurogenesis and cognitive improvement in a model of cholinergic denervation. Neuropharmacology (in press)
Vannucchi MG, Goldman-Rakic PS (1991) Age-dependent decrease in the affinity of muscarinic M1 receptors in neocortex of rhesus monkeys. Proc Natl Acad Sci USA 88(24):11475–11479
Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, VanEsen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME (2007) Intrinsic functional architecture in the anaesthetized monkey brain. Nature 447:83–86
Wagster MV, Whitehouse PJ, Walker LC, Kellar KJ, Price DL (1990) Laminar organization and age-related loss of cholinergic receptors in temporal neocortex of rhesus monkey. J Neurosci 10(9):2879–2885
Wamsley JK, Zarbin MA, Birdsall NJM, Kuhar MJ (1980) Muscarinic cholinergic receptors: autoradiographic localization of high and low affinity agonist binding sites. Brain Res 200:1–12
Wink AM, Bernard F, Salvador R, Bullmore E, Suckling J (2006) Age and cholinergic effects on hemodynamics and functional coherence of human hippocampus. Neurobiol Aging 27:1395–1404
Wise RG, Tracey I (2006) The role of fMRI in drug discovery. J Magn Reson Imaging 23(6):862–876
Wisman LA, Sahin G, Maingay M, Leanza G, Kirik D (2008) Functional convergence of dopaminergic and cholinergic input is critical for hippocampus-dependent working memory. J Neurosci 28(31):7797–7807
Zhang Z, Andersen AH, Ai Y, Loveland A, Hardy PA, Gerhardt GA, Gash DM (2006) Assessing nigrostriatal dysfunctions by pharmacological MRI in Parkinsonian rhesus macaques. Neuroimage 33(2):636–643
Acknowledgments
The authors would like to thank Dominique Eghlidi, Sharon Kryger, Laurie Renner, Alison Weiss, and Vince Warren for their technical assistance. This work was supported by NIH grants RR-000163, AG-023477, and AG-029612 and an OHSU Tartar Fellowship.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Haley, G.E., Kroenke, C., Schwartz, D. et al. Hippocampal M1 receptor function associated with spatial learning and memory in aged female rhesus macaques. AGE 33, 309–320 (2011). https://doi.org/10.1007/s11357-010-9184-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-010-9184-2