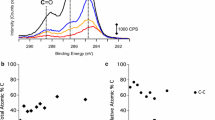
Abstract
Purpose
Precipitation of dissolved organic matter (DOM) by multivalent cations is important for biogeochemical cycling of organic carbon. We investigated to which extent cation bridges are involved in DOM precipitation and how cross-links by cations and water molecule bridges (WaMB) stabilise the matrix of precipitated DOM.
Materials and methods
DOM was precipitated from the aqueous extract of a forest floor layer adding solutions of Ca(NO3)2, Al(NO3)3 and Pb(NO3)2 with different initial metal cation/C (Me/C) ratios. Precipitates were investigated by differential scanning calorimetry before and after ageing to detect cation bridges, WaMB and restructuring of supramolecular structure.
Results and discussion
Twenty-five to sixty-seven per cent of the dissolved organic carbon was precipitated. The precipitation efficiency of cations increased in the order Ca < Al < Pb, while the cation content of precipitates increased in the order Pb < Ca < Al. The different order and the decrease in the WaMB transition temperature (T*) for Al/C > 3 is explained by additional formation of small AlOOH particles. Thermal analysis indicated WaMB and their disruption at T* of 53–65 °C. Like cation content, T* increased with increasing Me/C ratio and in the order Ca < Pb < Al for low Me/C. This supports the general assumption that cross-linking ability increases in the order Ca < Pb < Al. The low T* for high initial Me/C suggests less stable and less cross-linked precipitates than for low Me/C ratios.
Conclusions
Our results suggest a very similar thermal behaviour of OM bound in precipitates compared with soil organic matter and confirms the relevance of WaMB in stabilisation of the supramolecular structure of cation-DOM precipitates. Thus, stabilisation of the supramolecular structure of the DOM precipitates is subjected to dynamics in soils.





Similar content being viewed by others
References
Aquino AJA, Tunega D, Schaumann GE, Haberhauer G, Gerzabek MH, Lischka H (2011) The functionality of cation bridges for binding polar groups in soil aggregates. Int J Quantum Chem 111:1531–1542
Baldock JA, Skjemstad JO (2000) Role of the soil matrix and minerals in protecting natural organic materials against biological attack. Org Geochem 31:697–710
Bardy M et al (2007) Al speciation in tropical podzols of the upper Amazon basin: a solid-state Al-27 MAS and MQMAS NMR study. Geochim Cosmochim Acta 71:3211–3222
Borggaard OK, Raben-Lange B, Gimsing AL, Strobel BW (2005) Influence of humic substances on phosphate adsorption by aluminium and iron oxides. Geoderma 127:270–279
Cao XD, Ma LQ, Chen M, Hardison DW, Harris WG (2003) Weathering of lead bullets and their environmental effects at outdoor shooting ranges. J Environ Qual 32:526–534
Diehl D, Ellerbrock RH, Schaumann GE (2009) DRIFT-spectroscopy of untreated and dried soil samples of different wettability. Eur J Soil Sci 60:557–566
Dudal Y, Holgado R, Maestri G, Guillon E, Dupont L (2006) Rapid screening of DOM’s metal-binding ability using a fluorescence-based microplate assay. Sci Total Environ 354:286–291
Gustaffson JP (2010) Visual MINTEQ, version 3.0. http://www.lwr.kth.se/English/Oursoftware/vminteq/index.htm. Accessed 08 January 2014
Hurrass J, Schaumann GE (2005) Is glassiness a common characteristic of soil organic matter? Environ Sci Technol 39:9534–9540
Hurraß J, Schaumann GE (2006) Properties of soil organic matter and aqueous extracts of actually water repellent and wettable soil samples. Geoderma 132:222–239
Jäger A, Schaumann GE, Bertmer M (2011) Optimized NMR spectroscopic strategy to characterize water dynamics in soil samples. Org Geochem 42:917–925
Kalbitz K, Kaiser K (2008) Contribution of dissolved organic matter to carbon storage in forest mineral soils. J Plant Nutr Soil Sci Z Pflanzenernahr Bodenkunde 171:52–60
Khare N, Hesterberg D, Martin JD (2005) XANES investigation of phosphate sorption in single and binary systems of iron and aluminum oxide minerals. Environ Sci Technol 39:2152–2160
Klitzke S, Lang F, Kaupenjohann M (2008) Increasing pH releases colloidal lead in a highly contaminated forest soil. Eur J Soil Sci 59:265–273
Kunhi Mouvenchery Y, Kučerík J, Diehl D, Schaumann GE (2012) Cation-mediated cross-linking in natural organic matter—a review. Rev Environ Sci Biotechnol 11:41–54
Kunhi Mouvenchery Y, Jaeger A, Aquino AJA, Tunega D, Diehl D, Bertmer M, Schaumann GE (2013) Restructuring of a peat in interaction with multivalent cations: effect of cation type and aging time. PLoS ONE 8:e65359
Lang F, Egger H, Kaupenjohann M (2005) Size and shape of lead-organic associations. Colloid Surf A 265:95–103
LeBoeuf EJ, Weber WJ (2000) Macromolecular characteristics of natural organic matter. 1. Insights from glass transition and enthalpic relaxation behavior. Environ Sci Technol 34:3623–3631
Lu YF, Allen HE (2002) Characterization of copper complexation with natural dissolved organic matter (DOM)—link to acidic moieties of DOM and competition by Ca and Mg. Water Res 36:5083–5101
Miikki V, Senesi N, Hanninen K (1997) Characterization of humic material formed by composting of domestic and industrial biowastes.2. Spectroscopic evaluation of humic acid structures. Chemosphere 34:1639–1651
Mulder J, De Wit HA, Boonen HWJ, Bakken LR (2001) Increased levels of aluminium in forest soils: effects on the stores of soil organic carbon. Water Air Soil Pollut 130:989–994
Oste LA, Temminghoff EJM, Riemsdijk WH (2002) Solid-solution partitioning of organic matter in soils as influenced by an increase in pH or Ca concentration. Environ Sci Technol 36:208–214
Piccolo A (2002) The supramolecular structure of humic substances: a novel understanding of humus chemistry and implications in soil science. Adv Agron 75:57–134
Plaschke M, Rothe J, Denecke MA, Fanghanel T (2004) Soft X-ray spectromicroscopy of humic acid europium(III) complexation by comparison to model substances. J Electron Spectrosc Relat Phenom 135:53–62
Plaschke M, Rothe J, Armbruster MK, Denecke MA, Naber A, Geckeis H (2010) Humic acid metal cation interaction studied by spectromicroscopy techniques in combination with quantum chemical calculations. J Synchrotron Radiat 17:158–165
Römkens P, Dolfing J (1998) Effect of Ca on the solubility and molecular size distribution of DOC and Cu binding in soil solution samples. Environ Sci Technol 32:363–369
Schaumann GE (2005) Matrix relaxation and change of water state during hydration of peat. Colloid Surf A 265:163–170
Schaumann GE (2006) Soil organic matter beyond molecular structure. 2. Amorphous nature and physical aging. J Plant Nutr Soil Sci 169:157–167
Schaumann GE, Bertmer M (2008) Do water molecules bridge soil organic matter molecule segments? Eur J Soil Sci 59:423–429
Schaumann GE, Bertmer M (2013) Soil-water interactions. eMagRes 2:493–502
Schaumann GE, LeBoeuf EJ (2005) Glass transitions in peat—their relevance and the impact of water. Environ Sci Technol 39:800–806
Schaumann GE, Thiele-Bruhn S (2011) Molecular modelling of soil organic matter: squaring the circle? Geoderma 169:55–68
Schaumann GE, Siewert C, Marschner B (2000) Kinetics of the release of dissolved organic matter (DOM) from air-dried and pre-moistened soil material. J Plant Nutr Soil Sci 163:1–5
Schaumann GE, Diehl D, Bertmer M, Jaeger A, Conte P, Alonzo G, Bachmann J (2013a) Combined proton NMR wideline and NMR relaxometry to study SOM-water interactions of cation-treated soils. J Hydrol Hydromech 61:50–63
Schaumann GE, Gildemeister D, Kunhi Mouvenchery Y, Spielvogel S, Diehl D (2013b) Interactions between cations and water molecule bridges in soil organic matter. J Soils Sediments 13:1579–1588
Scheel T, Dorfler C, Kalbitz K (2007) Precipitation of dissolved organic matter by aluminum stabilizes carbon in acidic forest soils. Soil Sci Soc Am J 71:64–74
Scheel T, Haumaier L, Ellerbrock RH, Ruhlmann J, Kalbitz K (2008) Properties of organic matter precipitated from acidic forest soil solutions. Org Geochem 39:1439–1453
Schwesig D, Kalbitz K, Matzner E (2003) Effects of aluminium on the mineralization of dissolved organic carbon derived from forest floors. Eur J Soil Sci 54:311–322
Shen YH (1999) Sorption of humic acid to soil: the role of soil mineral composition. Chemosphere 38:2489–2499
Weng LP, Temminghoff EJM, Lofts S, Tipping E, Van Riemsdijk WH (2002) Complexation with dissolved organic matter and solubility control of heavy metals in a sandy soil. Environ Sci Technol 36:4804–4810
Wershaw RL (2004) Evaluation of conceptual models of natural organic matter (Humus) from a consideration of the chemical and biochemical processes of humification U.S. Department of the Interior, U.S. Geological Survey Reston, Virginia
Xia K, Bleam W, Helmke PA (1997) Studies of the nature of binding sites of first row transition elements bound to aquatic and soil humic substances using X-ray absorption spectroscopy. Geochim Cosmochim Acta 61:2223–2235
Acknowledgements
This study was financially supported by the Deutsche Forschungsgemeinschaft (DFG), project SCHA849/6. We further acknowledge the help of Petra Ziegler, group of Sören Thiele-Bruhn, University Trier, for the measurement of the Al concentrations.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Dong-Mei Zhou
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 275 kb)
Rights and permissions
About this article
Cite this article
Gildemeister, D., Metreveli, G., Spielvogel, S. et al. Stabilisation of precipitates of pedogenic dissolved organic matter by multivalent cations. J Soils Sediments 15, 1–12 (2015). https://doi.org/10.1007/s11368-014-0946-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-014-0946-9