Abstract
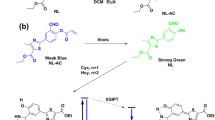
Glutathione (GSH) plays a critical role in maintaining oxidation-reduction homeostasis in biological systems. Considering the detection of GSH by fluorescence sensors is limited by either the short wavelength emission or the poor photostability, a highly stable colorimetric and ratiometric NIR fluorescent sensor (DCM-S) for GSH detection has been constructed on the basis of dicyanomethylene-4H-pyran (DCM) chromophore. The specific disulfide bond is incorporated via a carbamate linker as the GSH responsive group, which simultaneously blue-shifts and quenches the fluorescence. Upon addition of GSH, DCM-S exhibits outstanding colorimetric (from yellow to red) and ratiometric fluorescent response with the 6-fold enhancement of NIR fluorescence at 665 nm in quantum yield. More importantly, the GSH-treated DCM-S (DCM-NH2 actually) possesses 20-fold longer fluorescence half-life period as well as much better photostability than the FDA-approved ICG. Finally, the ratiometric detection of GSH is also successfully operated in the living cell imaging, exhibiting NIR fluorescence and large Stokes shift (215 nm) with nearly no background fluorescence interference. As a consequence, DCM-S can be utilized as colorimetric and ratiometric NIR fluorescent sensor for GSH, with a great potential in the development of GSH-induced drug delivery system.
Similar content being viewed by others
References
Chen XQ, Zhou Y, Peng XJ, Yoon J. Chem Soc Rev, 2010, 39: 2120–2135
Hou YC, Guo ZM, Li J, Wang PG. Biochem Biophys Res Commun, 1996, 228: 88–93
Wu XM, Sum XR, Guo ZQ, Tang JB, Shen YQ, James TD, Tian H, Zhu WH. J Am Chem Soc, 2014, 136: 3579–3588
Wood ZA, Schröder E, Harris JR, Poole LB. Trends Biochem Sci, 2003, 28: 32–40
Finkel T, Holbrook NJ. Nature, 2000, 408: 239–247
Hou SG, Liang L, Deng SSH, Chen JF, Huang Q, Cheng Y, Fan CH. Sci China Chem, 2014, 57: 100–106
Kizek R, Vacek J, Trnková L, Jelen F. Bioelectrochemistry, 2004, 63: 19–24
Feng LH, Liu LB, Lv FT, Bazan GC, Wang S. Adv Mater, 2014, 26: 3926–3930
Vacek J, Klejdus B, Petrlová J, Lojková L, Kubán V. Analyst, 2006, 131: 1167–1174
Ling YY, Yin XF, Fang ZL. Electrophoresis, 2005, 26: 4759–4766
Satoh I, Arakawa S, Okamoto A. Sens Actuators B, 1991, 5: 245–247
Pastore A, Federici G, Bertini E, Piemonte F. Clin Clim Acta, 2003, 333: 19–39
Li K, Yu X, Tong AJ, Tang BZ. Sci China Chem, 2014, 57: 248–251
Miao QQ, Li Q, Yuan QP, Li LL, Hai ZJ, Liu S, Liang GL. Anal Chem, 2015, 87: 3460–3466
Zheng MM, Huang HX, Zhou M, Wang YQ, Zhang Y, Ye DJ, Chen HY. Chem Eur J, 2015, 21: 10506–10512
Yin J, Kwon Y, Kim D, Lee D, Kim G, Hu Y, Ryu JH, Yoon J. J Am Chem Soc, 2014, 136: 5351–5358
Lee MH, Han JH, Kwon P, Bhuniya S, Kim JY, Sessler JL, Kang C, Kim JS. J Am Chem Soc, 2012, 134: 1316–1322
Lou ZR, Li P, Sun XF, Yang SQ, Wang BS, Han KL. Chem Commun, 2013, 49: 391–393
Long LL, Lin WY, Chen BB, Gao WS, Yuan L. Chem Commun, 2011, 47: 893–895
Niu LY, Guan YS, Chen YZ, Wu LZ, Tung CH, Yang QZ. J Am Chem Soc, 2012, 134: 18928–18931
Wang FY, Zhou L, Zhao CC, Wang R, Fei Q, Luo SH, Guo ZQ, Tian H, Zhu WH. Chem Sci, 2015, 6: 2584–2589
Jung HS, Ko KC, Kim GH, Lee AR, Na YC, Kang C, Lee JY, Kim JS. Org Lett, 2011, 13: 1498–1501
Sreejith S, Divya KP, Ajayaghosh A. Angew Chem Int Ed, 2008, 47: 7883–7887
Ding J, Li HY, Wang C, Yang J, Xie YJ, Peng Q, Li QQ, Li Z. ACS Appl Mater Interf, 2015, 7: 11369–11376
Frangioni JV. Curr Opin Chem Biol, 2003, 7: 626–634
Majumdar P, Yuan XL, Li SF, Guennic BL, Ma J, Zhang CS, Jacqueminde D, Zhao JZ. J Mater Chem B, 2014, 2: 2838–2854
Li YH, Sun Y, Li JC, Su QQ, Yuan W, Dai Y, Han CM, Wang QH, Feng W, Li FY. J Am Chem Soc, 2015, 137: 6407–6416
Sun W, Fan JL, Hu C, Cao JF, Zhang H, Xiong XQ, Wang JY, Cui S, Sun SG, Peng XJ. Chem Commun, 2013, 49: 3890–3892
Wu XM, Chang S, Sun XR, Guo ZQ, Li YS, Tang JB, Shen YQ, Shi JL, Tian H, Zhu WH. Chem Sci, 2013, 4: 1221–1228
Samanta A, Vendrell M, Das R, Chang YT. Chem Commun, 2010, 46: 7406–7408
Shank NI, Pham HH, Waggoner AS, Armitage BA. J Am Chem Soc, 2013, 135: 242–251
Altman RB, Zheng QS, Zhou Z, Terry DS, Warren JD, Blanchard SC. Nat Method, 2012, 9: 428–429
Barbon A, Bott ED, Brustolon M, Fabris M, Kahr B, Kaminsky W, Reid PJ, Wong SM, Wustholz KL, Zanré R. J Am Chem Soc, 2009, 131: 11548–11557
Guo ZQ, Zhu WH, Shen LJ, Tian H. Angew Chem Int Ed, 2007, 46: 5549–5553
Guo ZQ, Zhu WH, Xiong YY, Tian H. Macromolecules, 2009, 42: 1448–1453
Liu B, Li XY, Liu MY, Ning ZJ, Zhang Q, Li C, Müllen K, Zhu WH. Dyes Pigm, 2012, 94: 23–27
Guo ZQ, Zhu WH, Tian H. Chem Commun, 2012, 48: 6073–6084
Zhu WH, Huang XM, Guo ZQ, Wu XM, Yu HH, Tian H. Chem Commun, 2012, 48: 1784–1786
Lee MH, Yang ZG, Lim CW, Lee YH, Dongbang S, Kang C, Kim JS. Chem Rev, 2013, 113: 5071–5109
Li M, Wu XM, Wang Y, Li YS, Zhu WH, James TD. Chem Commun, 2014, 50: 1751–1753
Yang ZG, Lee JH, Jeon HM, Han JH, Park N, He YX, Lee H, Hong KS, Kang C, Kim JS. J Am Chem Soc, 2013, 135: 11657–11662
Mahato R, Tai W, Cheng K. Adv Drug Delivery Rev, 2011, 63: 659–670
Belsky I, Dodiuk H, Shvo Y. J Org Chem, 1974, 39: 989–995
Hammond PR. Opt Commun, 1979, 29: 331–333
Chung C, Srikun D, Lim CS, Chang CJ, Cho BR. Chem Commun, 2011, 47: 9618–9620
Lim CS, Masanta G, Kim HJ, Han JH, Kim HM, Cho BR. J Am Chem Soc, 2011, 133: 11132–11135
He XX, Wang YS, Wang KM, Chen M, Chen SY. Anal Chem, 2012, 84: 9056–9064
Wang G, Chang XM, Peng JX, Liu KQ, Zhao K, Yu CM, Fang Y. Phys Chem Chem Phys, 2015, 17: 5441–5449
Guo T, Cui L, Shen JN, Wang R, Zhu WP, Xu YF, Qian XH. Chem Commun, 2013, 49: 1862–1864
Altman RB, Terry DS, Zhou Z, Zheng QS, Geggier P, Kolster RA, Zhao YF, Javitch JA, Warren JD, Blanchard SC. Nat Method, 2012, 9: 68–71
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wu, X., Shao, A., Zhu, S. et al. A novel colorimetric and ratiometric NIR fluorescent sensor for glutathione based on dicyanomethylene-4H-pyran in living cells. Sci. China Chem. 59, 62–69 (2016). https://doi.org/10.1007/s11426-015-5490-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-015-5490-y