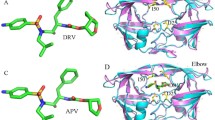
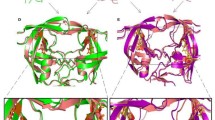
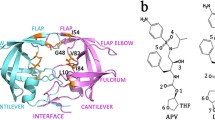
Abstract
Although HIV-1 subtype B still dominates the epidemic AIDS in developed countries, an increasing number of people in developing countries are suffering from an epidemic of non-subtype B viruses. What is worse, the efficacy of the combinational use of antiretroviral drugs is gradually compromised by the rapid development of drug resistance. To gain an insight into drug resistance, 10-ns MD simulations were simultaneously conducted on the complexes of the TL-3 inhibitor with 4 different proteases (B wt, B mut, F wt and F mut), among which the complex of the B wt protease with the TL-3 inhibitor was treated as the control group. Detailed analyses of MD data indicated that the drug resistance of B mut against TL-3 mainly derived from loss of an important hydrogen bond and that of F wt was caused by the decrease of hydrophobic interactions in S1/S1’ pocket, while both of the two reasons mentioned above were the cause of the F mut protease’s resistance. These results are in good agreement with the previous experiments, revealing a possible mechanism of drug resistance for the aforementioned protease subtypes against the TL-3 inhibitor. Additionally, another indication was obtained that the mutations of M36I, V82A and L90M may induce structural transforms so as to alter the inhibitor’s binding mode.
Similar content being viewed by others
References
UNAIDS. Annual Report 2009, http://data.unaids.org/pub/Report/2010/2009_annual_report_en.pdf
Caride E, Hertogs K, Larder B, et al. Genotypic and phenotypic evidence of different drug-resistance mutation patterns between B and non-B subtype isolates of human immunodeficiency virus type 1 found in Brazilian patients failing HAART. Virus Genes, 2001, 23: 193–202
Foulkes-Murzycki J E, Scott W R, Schiffer C A. Hydrophobic sliding: A possible mechanism for drug resistance in human immunodeficiency virus type 1 protease. Structure, 2007, 15: 225–233
Abramowitz N, Schechter I, Berger A. On the size of the active site in protease. II. Carboxypeptidase-A. Biochem Biophys Res Commun, 1967, 29: 862–867
Schechter I, Berger A. On the size of the active site in protease. I. Papain. Biochem Biophys Res Commun, 1967, 27: 157–162
DeGruttola V, Dix L, Aquila D R, et al. The relation between baseline HIV drug resistance and response to antiretroviral therapy: Re-analysis of retrospective and prospective studies using a standardized data analysis plan. Antivir Ther, 2000, 5: 41–48
Ledergerber B, Egger M, Erard V, et al. AIDS-related opportunistic illnesses occurring after initiation of potent antiretroviral therapy: The Swiss HIV cohort study. Jama, 1999, 82: 2220–2226
Sanches M, Krauchenco S, Martins N H, et al. Structural characterization of B and non-B subtypes of HIV-protease: Insights into the natural susceptibility to drug resistance development. J Mol Biol, 2007, 369: 1029–1040
Case D A, Pearlman D A, Caldwell J W, et al. AMBER 8. San Francisco: University of California, 2004
Kollman P A, Cornell W D, Chipot C, et al. The development/application of “minimalist”organic/biochemical molecular mechanic force field using a combination of ab initio calculations and experimental data. Comp Simulat Biolog Syst, 1997, 3: 83–96
Jorgensen W L, Madura J D, Impey R W, et al. Comparison of simple potential functions for simulating liquid water. J Chem Phys, 1983, 79: 926–935
Wang J, Wolf R M, Caldwell J W, et al. Development and testing of a general amber force field. J Comput Chem, 2004, 25: 1157–1174
Rychaert J P, Berendsen H J C. Numerical integration of Cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. Comput Phys, 1977, 23: 327–341
Darden T, Pedersen L. Particle mesh Ewald: An N-log(N) method for Ewald sums in large systems. J Chem Phys, 1993, 98: 10089–10092
Wang J F, Wei D Q, Li L, et al. 3D structure modeling of cytochrome P450 2C19 and its implication for personalized drug design. Biochem Biophys Res Commun, 2007, 355: 513–519
Wang J F, Wei D Q, Lin Y, et al. Insights from modeling the 3D structure of NAD(P)H-dependent D-xylose reductase of Pichia stipitis and its binding interactions with NAD and NADP. Biochem Biophys Res Commun, 2007, 359: 323–329
Wang J F, Wei D Q, Chen C, et al. Molecular modeling of two CYP2C19 SNPs and its implications for personalized drug design. Protein Pept Lett, 2008, 15: 27–32
Wang J F, Wei D Q, Chou K C. Pharmacogenomics and personalized use of drugs. Curr Top Med Chem, 2008, 8: 1573–1579
Wang J F, Wei D Q, Chou K C. Drug candidates from traditional Chinese medicine. Curr Top Med Chem, 2008, 8: 1656–1665
Wang J F, Zhang C C, Chou K C, et al. Structure of cytochrome P450s and personalized drug. Curr Med Chem, 2009, 16: 232–244
Wang J F, Gong K, Wei D Q, et al. Molecular dynamics studies on the interactions of PTP1B with inhibitors: From the first phosphate-binding site to the second one. Protein Eng Des Sel, 2009, 22: 349–355
Wang J F, Wei D Q, Chou K C. Insights from investigating the interactions of adamantane-based drugs with the M2 proton channel from the H1N1 swine virus. Biochem Biophys Res Commun, 2009, 388: 413–417
Wang J F, Chou K C. Insights into the molecular switch mechanism of human Rab5a from molecular dynamics simulations. Biochem Biophys Res Commun, 2009, 390: 608–612
Wallace A C, Laskowski R A, Thornton J M. LIGPLOT: a program to generate schematic diagrams of protein-ligand interactions. Protein Eng, 1995, 8: 127–134
Hansch C. Quantitative structure-activity relationships and the unnamed science. Acc Chem Res, 1993, 26: 147–153
Hansch C, Hoekman D, Gao H. Comparative QSAR: Toward a deeper understanding of chemicobiological interactions. Chem Rev, 1996, 96: 1045–1076
Physicians Desk Reference. Montvale, USA: Medical Ergonomics Data Production Company, 1995
Hong L, Zheng X C, Hartsuck J A, et al. Crystal structure of an in vivo HIV-1 protease mutant in complex with saquinavir: Insights into the mechanism of drug resistance. Protein Sci, 2000, 9: 1898–1904
Author information
Authors and Affiliations
Corresponding authors
About this article
Cite this article
Gu, H., Chen, H., Wei, D. et al. Molecular dynamics simulations exploring drug resistance in HIV-1 proteases. Chin. Sci. Bull. 55, 2677–2683 (2010). https://doi.org/10.1007/s11434-010-3257-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-010-3257-6