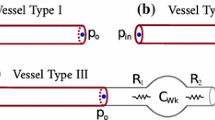
Abstract
A computational model of the entire cardiovascular system is established based on multi-scale modeling, where the arterial tree is described by a one-dimensional model coupled with a lumped parameter description of the remainder. The resultant multi-scale model forms a closed loop, thus placing arterial wave propagation into a global hemodynamic environment. The model is applied to study the global hemodynamic influences of aortic valvular and arterial stenoses located in various regions. Obtained results show that the global hemodynamic influences of the stenoses depend strongly on their locations in the arterial system, particularly, the characteristics of hemodynamic changes induced by the aortic valvular and aortic stenoses are pronounced, which imply the possibility of noninvasively detecting the presence of the stenoses from peripheral pressure pulses. The variations in aortic pressure/flow pulses with the stenoses play testimony to the significance of modeling the entire cardiovascular system in the study of arterial diseases.






Similar content being viewed by others
References
Alastruey J, Parker KH, Peiró J et al (2007) Modelling the circle of Willis to assess the effects of anatomical variations and occlusions on cerebral flows. J Biomech 40:1794–1805. doi:10.1016/j.jbiomech.2006.07.008
Arani DT, Carleton RA (1967) Assessment of aortic valvular stenosis from the aortic pressure pulse. Circulation 36:30–35
Bertolotti C, Qin Z, Lamontagne B et al (2006) Influence of multiple stenosis on Echo–Doppler functional diagnosis of peripheral arterial disease: a numerical and experimental study. Ann Biomed Eng 34:564–574. doi:10.1007/s10439-005-9071-7
Feringa HHH, Bax JJJ, van Waning VH et al (2006) The long-term prognostic value of the resting and postexercise ankle-brachial index. Arch Intern Med 166:529–535. doi:10.1001/archinte.166.5.529
Fitchett DH (1991) LV-arterial coupling: interactive model to predict effect of wave reflections on LV energetics. Am J Physiol 261:H1026–H1033
Formaggia L, Nobile F, Quarteroni A et al (1999) Multiscale modeling of the circulatory system: a preliminary analysis. Comput Vis Sci 2:75–83. doi:10.1007/s007910050030
Formaggia L, Lamponi D, Tuveri M, Veneziani A (2006) Numerical modeling of 1-D arterial networks coupled with a lumped parameters description of the heart. Comput Methods Biomech Biomed Eng 9:273–288. doi:10.1080/10255840600857767
Garcia D, Barenbrug PJC, Pibarot P et al (2005) A ventricular-vascular coupling model in presence of aortic stenosis. Am J Physiol Heart Circ Physiol 288:H1874–H1884. doi:10.1152/ajpheart.00754.2004
Garcia D, Pibarot P, Durand LG (2005) Analytical modeling of the instantaneous pressure gradient across the aortic valve. J Biomech 38:1303–1311. doi:10.1016/j.jbiomech.2004.06.018
Garcia D, Durand LG (2006) Modeling of aortic stenosis and systemic hypertension. In: Akay M (eds) Wiley encyclopedia of biomedical engineering. Wiley, New York. doi: 10.1002/9780471740360.ebs1527
He Y, Liu H, Himeno R (2004) A one-dimensional thermo-fluid model of blood circulation in the human upper limb. Int J Heat Mass Transf 47:2735–2745. doi:10.1016/j.ijheatmasstransfer.2003.10.041
Heldt T, Shim EB, Kamm RD, Mark RG (2002) Computational modeling of cardiovascular response to orthostatic stress. J Appl Physiol 92:1239–1254
Heusden KV, Gisolf J, Stok WJ et al (2006) Mathematical modeling of gravitational effects on the circulation: importance of the time course of venous pooling and blood volume changes in the lungs. Am J Physiol 291:H2152–H2165
Kass DA (2005) Ventricular arterial stiffening: integrating the pathophysiology. Hypertension 46:1–9. doi:10.1161/01.HYP.0000168053.34306.d4
Liang FY, Liu H (2005) A closed-loop lumped parameter computational model for human cardiovascular system. JSME Int J 48(C):484–493. doi:10.1299/jsmec.48.484
Liang FY, Liu H (2006) Simulation of hemodynamic responses to the Valsalva maneuver: an integrative computational model of the cardiovascular system and the autonomic nervous system. J Physiol Sci 56:45–65. doi:10.2170/physiolsci.RP001305
Lorell BH, Carabello BA (2000) Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation 102:470–479
Melis MD, Morbiducci U, Rietzschel ER et al (2008) Blood pressure waveform analysis by means of wavelet transform. Med Biol Eng Comput (in press). doi:10.1007/s11517-008-0397-9
Mohrman DE, Heller LJ (2006) Cardiovascular physiology, 6th edn. McGraw-Hill, New York
Olufsen M (1998) Modeling the arterial system with reference to an anesthesia simulator. PhD Thesis, Rotskilde University
Pontrelli G (2002) A mathematical model of flow in a liquid-filled visco-elastic tube. Med Biol Eng Comput 40:550–556. doi:10.1007/BF02345454
Pontrelli G (2004) A multiscale approach for modeling wave propagation in an arterial segment. Comput Methods Biomech Biomed Eng 7:79–89. doi:10.1080/1025584042000205868
Seeley BD, Young DF (1976) Effect of geometry on pressure losses across models of arterial stenoses. J Biomech 9:439–448. doi:10.1016/0021-9290(76)90086-5
Sherwin SJ, Franke V, Peiró J et al (2003) One-dimensional modelling of a vascular network in space-time variables. J Eng Math 47:217–250. doi:10.1023/B:ENGI.0000007979.32871.e2
Siouffi M, Deplano V, Pélissier R (1998) Experimental analysis of unsteady flows through a stenosis. J Biomech 31:11–19. doi:10.1016/S0021-9290(97)00104-8
Smith FT, Ovenden NC, Franke PT et al (2003) What happens to pressure when a flow enters a side branch? J Fluid Mech 479:231–258. doi:10.1017/S002211200200366X
Stergiopulos N, Young DF, Rogge TR (1992) Computer simulation of arterial flow with applications to arterial and aortic stenoses. J Biomech 25:1477–1488. doi:10.1016/0021-9290(92)90060-E
Suga H, Sagawa K, Shoukas AA (1973) Load independence of the instantaneous pressure-volume ratio of the canine left ventricle and effects of epinephrine and heart rate on the ratio. Circ Res 32:314–322
Sun Y, Beshara M, Lucariello RJ, Chiaramida SA (1997) A comprehensive model for right-left heart interaction under the influence of pericardium and baroreflex. Am J Physiol 272:H1499–H1515
Wang JJ, Parker KH (2004) Wave propagation in a model of the arterial circulation. J Biomech 37:457–470. doi:10.1016/j.jbiomech.2003.09.007
Wang JJ, Shrive NG, Park KH et al (2008) ‘‘Wave’’ as defined by wave intensity analysis. Med Biol Eng Comput (in press). doi:10.1007/s11517-008-0403-2
Wild SH, Lee AJ, Byrne CD (2006) Low ankle-brachial pressure index predicts increased risk of cardiovascular disease independent of the metabolic syndrome and conventional cardiovascular risk factors in the Edinburgh artery study. Diabetes Care 29:637–642. doi:10.2337/diacare.29.03.06.dc05-1637
Young DF, Tsai F (1973) Flow characteristics in models of arterial stenoses II. Unsteady flow. J Biomech 6:547–559. doi:10.1016/0021-9290(73)90012-2
Acknowledgments
This research was supported by Research and Development of the Next-Generation Integrated Simulation of Living Matter, a part of the Development and Use of the Next-Generation Supercomputer Project of MEXT, Japan. We sincerely thank the reviewers for the helpful comments. Special thanks to P. L. Wilson for help in manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1: Numerical methods for bifurcation conditions
The system of Eqs. 4 and 5 describing the bifurcation conditions contains six unknowns, requiring another three equations to complete the system. We adopt a ‘ghost-point’ method [11] in which a “ghost” point is assumed to exist between the last two grid points of the mother tube and the first two grid points of the daughter tubes. When the current time is (n + 1)∆t, the index of the last grid-point of the mother tube is m, and that of the first grid point of the daughter tubes is 0, an algebraic equation can be obtained by discretizing the mass conservation equation at each of the three ghost-points.
For the mother tube:
and for the daughter tubes:
Substituting Eqs. 15 and 16 into Eqs. 4 and 5 yields a set of nonlinear coupled algebraic equations which may be solved by using an iterative Newton–Raphson method.
Appendix 2: Numerical methods for 0–1-D coupling
Several numerical methods have been proposed to handle 0–1-D coupling problems [6, 7, 22]. We developed a new 0–1-D coupling method better fitting the current model system. Our method starts by extrapolating Riemann invariants [7] on the 1-D model side.
Riemann invariants are the characteristic variables of a hyperbolic system (Eq. 17) transformed from Eq. 1.
where (W 1, W 2) are the Riemann invariants which represent, respectively, a forward-and a backward-traveling wave at speed, λ 1, λ 2. By choosing the reference conditions (A = A 0, U = 0), we can obtain the solutions of system (Eq. 17) as [7]
where c is the wave speed when A = A, and c 0 the wave speed when A = A 0.
From Eq. 18, we may rewrite (A, U) in terms of (W 1, W 2):
Since W 1, W 2 represent waves traveling at certain speeds, at a certain time step (t n+1), we may derive the values of W 1 (t n+1, L) and W 2 (t n+1, 0) from values at the previous time step (t n) at the distal end (denoted by L) and proximal end (denoted by 0) of an artery by extrapolating the outgoing Riemann invariants along the characteristic lines [7]
where ∆t is the time step.
Based on the extrapolated Riemann invariants, other unknowns can be derived from the interface conditions. Because analytical solutions to the interface conditions are not obtainable, numerical iteration is required to approximate the solutions. Taking the interface at the distal end of a peripheral artery as an example, the procedure of numerical iteration is described in Fig. 7, where t n+1 denotes the current time step, and t n+2 the next time step; W 1 (t n+1, L) is the extrapolated Riemann invariant; Q* and P* are the intermediate variables belonging commonly to the 0-D model and the 1-D model, and W 2*, U* and A* the intermediate variables of the 1-D model. The residual error is calculated based on Q*. All the intermediate variables will approximate the true solutions after the coupling computation converges.
Rights and permissions
About this article
Cite this article
Liang, F., Takagi, S., Himeno, R. et al. Multi-scale modeling of the human cardiovascular system with applications to aortic valvular and arterial stenoses. Med Biol Eng Comput 47, 743–755 (2009). https://doi.org/10.1007/s11517-009-0449-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-009-0449-9